The Gründer Lab
The aim of the Gründer lab is to characterize at the functional and structural level ion channels of the DEG/ENaC gene family. We strive to identify fundamental principles and to produce high-quality data that stand the test of time.

Our research has a focus on ion channels. Ion channels are the basis of neuronal excitability and of communication between neurons. Mutations in ion channels can lead to diseases and malregulation of ion channels determines the clinical picture of many diseases. The analysis of ion channels in molecular detail contributes to a better understanding of their pathophysiological role and is the basis for an educated pharmacotherapy.
Currently we have a focus on ion channels of the DEG/ENaC gene family. We are especially interested in 3 subfamilies, which contain closely related ion channels that are activated by 3 different extracellular ligands:
Acid-sensing ion channels (ASICs) are one of the major receptors for extracellular protons; they are broadly expressed in neurons of the central and peripheral nervous system. ASICs contribute to synaptic transmission and sensory transduction. ASICA pathophysiological role results from the fact that protons are also released during an ischemia; activation of ASICs thus contributes to angina pectoris and the cell death that accompanies stroke. In addition, it has been reported that ASICs contribute to axonal degeneration in an animal model for multiple sclerosis, and that they are critical for termination of seizures by acidosis. Thus, ASICs are of great physiological and pathophysiological importance. A detailed understanding of their activation and regulation are, thus, of great relevance.
3 relevant key publications of our group:
1) Chen X, Kalbacher H and Gründer S (2006) Interaction of Acid Sensing Ion Channel (ASIC) 1 with the tarantula toxin Psalmotoxin 1 is state dependent. J. Gen. Physiol., 127 (3), 267-276
2) Paukert M, Chen X, Polleichtner G, Schindelin H and Gründer S (2008) Candidate amino acids involved in H+ gating of acid-sensing ion channel (ASIC) 1a. J. Biol. Chem., 283, 572-581
3) Bartoi T, Augustinowski K, Polleichtner G, Gründer S, und Ulbrich MH (2014) Acid-sensing ion channel (ASIC) 1a/2a heteromers have a flexible 2:1/1:2 stoichimetry. Proc. Natl. Acad Sci., 111, 8281-8286
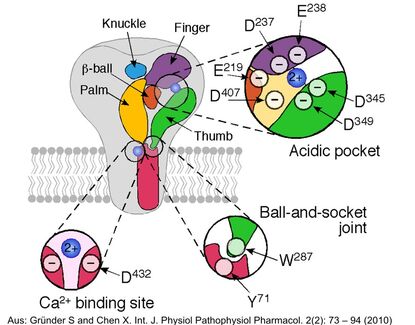
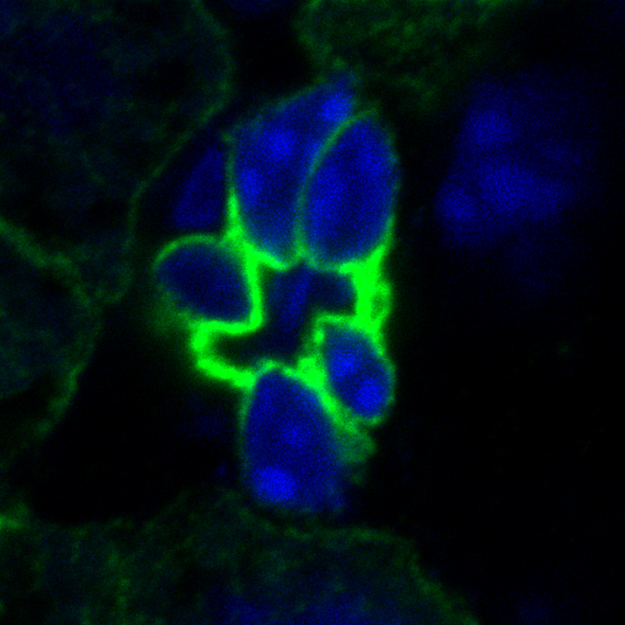
The bile acid-sensitive ion channel (BASIC) is a sparsely characterized ion channel that is expressed in bile ducts and that is directly activated by bile acids. In bile ducts and the small intestine, BASIC is potentially involved in Na+ transport. Therefore, BASIC might have potential impact for diarrhea andBASIC cholestasis. In addition, BASIC is present in brain, where he has an as yet completely unknown function.
3 relevant key publications of our group:
1) Wiemuth D and Gründer S (2010) A single amino acid tunes Ca2+ sensitivity of brain liver intestine Na+ channel (BLINaC). J. Biol. Chem., 285, 30404-30410 (2010)
2) Wiemuth D and Gründer S (2011) The pharmacological profile of brain liver intestine Na+ channel (BLINaC): inhibition by diarylamidines and activation by fenamates. Mol. Pharmacol., 80 (5), 911–919
3) Wiemuth D, Sahin H, Falkenburger BH, Lefèvre C, Wasmuth HE and Gründer S (2012) BASIC - a bile acid-sensitive ion channel highly expressed in bile ducts. FASEB J., 26, 4122-4130
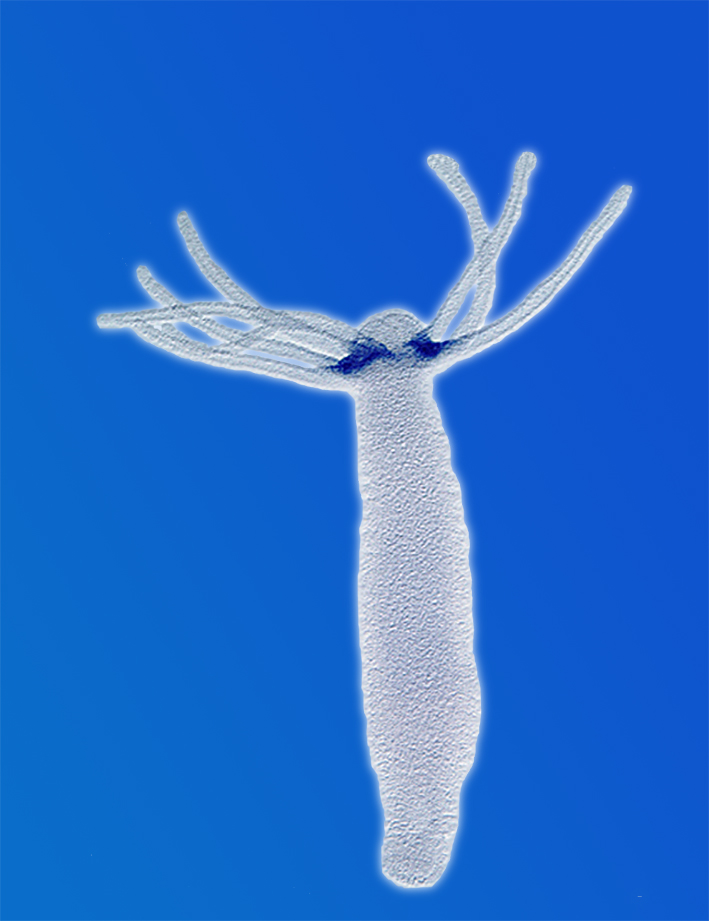
Peptid-gated ion channels are only sparsely investigated. The dogma holds that peptides only gate G-protein-coupled receptors. We could isolate a group of peptide-gated ion channels, the Hydra Na+ Channels (HyNaCs), from a simple model organism (the freshwater polyp Hydra magnipapillata), suggesting that neuropeptides were used for fast synaptic transmission already in simple nervous systems. Neuropeptides are common also in humans and ASICs are modulated by neuropeptides. We investigate HyNaCs as a model channel to better understand neurotransmission in a simple nervous system and to identify conserved features in the closely related ASICs and BASIC.
2 relevant key publications of our group:
1) Golubovic A, Kuhn A, Williamson M, Kalbacher H, Holstein TW, Grimmelikhuijzen CJ and Gründer S (2007) A peptide-gated ion channel from the freshwater polyp Hydra. J. Biol. Chem., 282, 35098-35103
2) Dürrnagel S, Kuhn A, Tsiairis CD, Williamson M, Kalbacher H, Grimmelikhuijzen CJ, Holstein TW and Gründer S (2010) Three homologous subunits form a high-affinity peptide-gated ion channel in Hydra. J. Biol. Chem., 285, 11958-11965
3) Assmann M, Kuhn A, Dürrnagel S, Holstein TW und Gründer S (2014) The comprehensive analysis of DEG/ENaC subunits in Hydra reveals a large variety of peptide-gated channels, potentially involved in neuromuscular transmission. BMC Biol., 12(1), 84

![[Translate to en:] [Translate to en:]](/fileadmin/files/institute/physiologie/_processed_/9/6/csm__ME18759_4dcd3c7cfd.jpg)
![[Translate to en:] [Translate to en:]](/fileadmin/files/institute/physiologie/_processed_/4/6/csm__ME18836_e9746e1c31.jpg)
![[Translate to en:] [Translate to en:]](/fileadmin/files/institute/physiologie/_processed_/d/e/csm__ME18866_8e2719aa71.jpg)
![[Translate to en:] [Translate to en:]](/fileadmin/files/institute/physiologie/_processed_/b/6/csm__ME18941_58dbfb528e.jpg)
![[Translate to en:] [Translate to en:]](/fileadmin/files/institute/physiologie/_processed_/0/7/csm__ME18965h_2b5a133f2a.jpg)
![[Translate to en:] [Translate to en:]](/fileadmin/files/institute/physiologie/_processed_/a/5/csm__ME18992_688dd41c10.jpg)
![[Translate to en:] [Translate to en:]](/fileadmin/files/institute/physiologie/_processed_/1/1/csm__ME19185_8a61b218cf.jpg)
![[Translate to en:] [Translate to en:]](/fileadmin/files/institute/physiologie/_processed_/7/5/csm__ME27611_14f178eb7a.jpg)