Research
Our research activity can be assigned into three main sections:
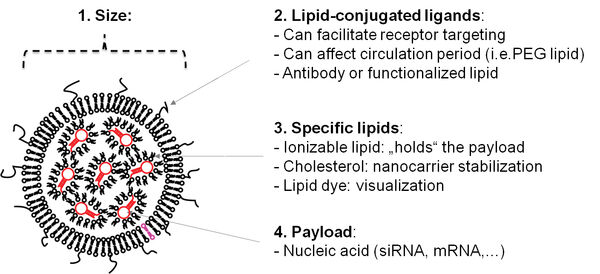
1. Encapsulation of nucleic acids using microfluidics technology
The first main focus of our research covers the usage of microfluidics technology to generate lipid nanocarriers for nucleic acids. In this regard, we use commercial solutions as well as self-constructed microchips. The result of the encapsulation are nanocarriers which can be administered in vivo. In recent years, the first two siRNA-based drugs have entered the market, and vaccinations based on mRNA have received tremendous attention in science and media.
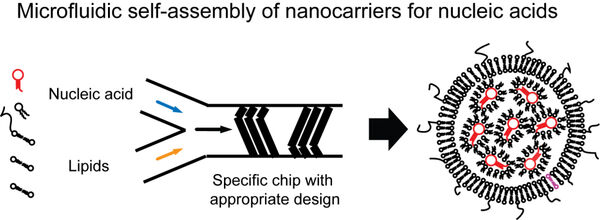
The lipid shell allows for further modifications such as fluorescent dyes which allow to trace the nanocarriers in fluorescence-based applications including non-invasive molecular imaging. Furthermore, tailored ligands can be incorporated that can enable to bind to cellular receptors.
2. Response of human cells and organ models to nanomaterials
The second focus and expertize of our group is given by studies on the nanocarriers using different types of human primary cells. We are focused on cells with immunological functions which comprise endothelial cells as well as immune cells. Ludwig Aschoff summarized the macrophages and endothelial cells as the „reticuloendothelial system“ (RES).
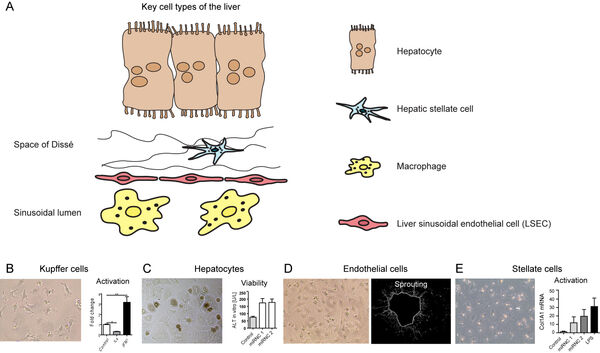
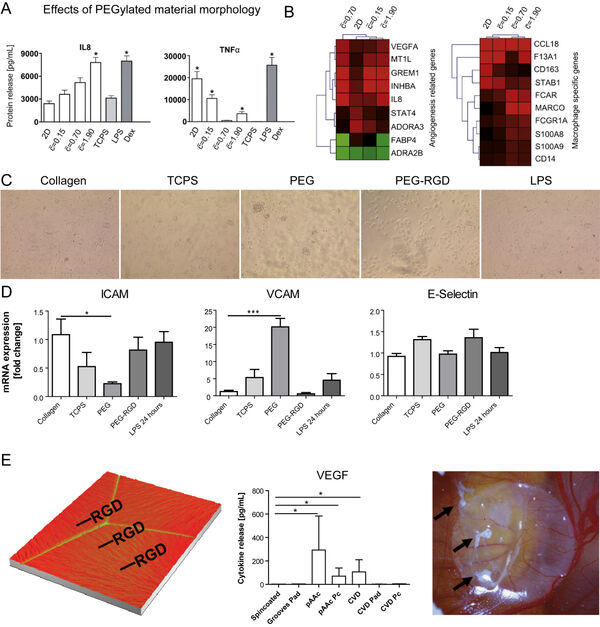
In earlier studies, we have investigated nanomaterial-induced activation of macrophages (Figure 4A-B) and liver-derived endothelial cells (Figure 4C-D). The activation of both cell types to materials is cell type-specific. Surface modifications of implants are frequently done using bioactive peptides. However, immune cells such as macrophages might evoke a rejection of an implant due to an undesired activation by the materials. Here, we study the influence of different strategies for peptide immobilization onto (poly)-vinylidene fluoride (PVDF) on inflammation and angiogenesis. Acrylic acid (AAc)-based covalent RGD-coupling leads to the most favorable cellular reaction, indicated by increased VEGF release, whereas covalent coupling of RGD induces a pronounced proinflammatory reaction (Figure 4E).
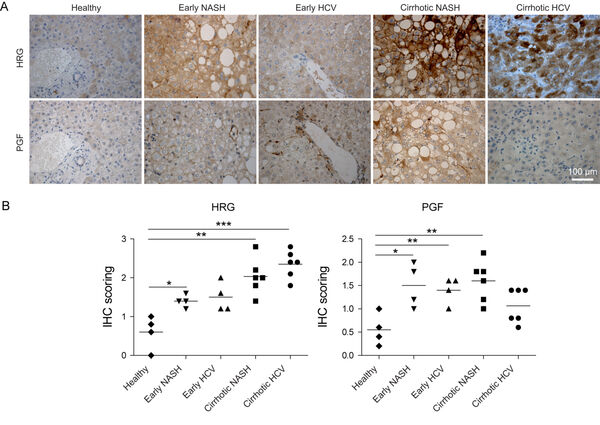
The in vivo signaling of endothelial cells and macrophages is highly specialized. While inflammatory macrophages i.e. release the histidine-rich glycoprotein, generation of novel blood vessels as reflected by expression of the placental growth factor (PGF) occurrs at different regions as demonstrated in serial sections. PGF signaling is significantly increased in non-alcoholic steatohepatitis (NASH), and in early hepatitis C virus patients (Figure 5A,B).
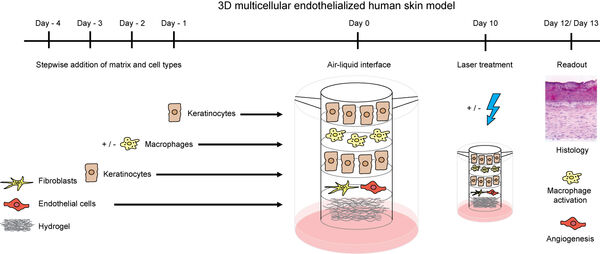
We further study additional cell types of other organs. We study human-derived cellular monocultures in order to receive a clear response of specific single cell types. Particularly in the context of advanced 3D models with multicellular human primary cell models, we employ other parenchymal cells types.
3. In vivo studies of small molecules, nanoparticles, and nucleic acid-based drugs
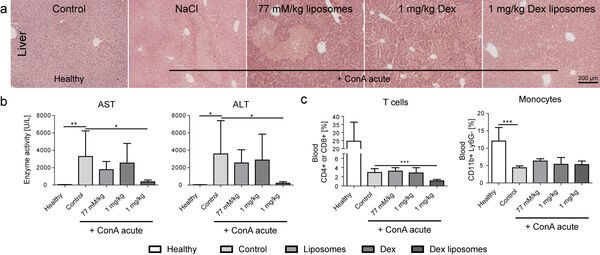
Our studies form 2014/2015 have demonstrated that dexamethasone-loaded lipid-based nanocarriers are efficient in treating acute liver disease (Figure 7A,B). However, the depletion of T cells represented a key side effect of the otherwise efficient treatment (Figure 7C).
Selectins are the communicative interface between immune cells and the endothelium. Selectins and their ligands are regulated during inflammatory processes by both cell types. We have demonstrated that selectin inhibition is capable to ameliorate acute liver diease (Figure 8).
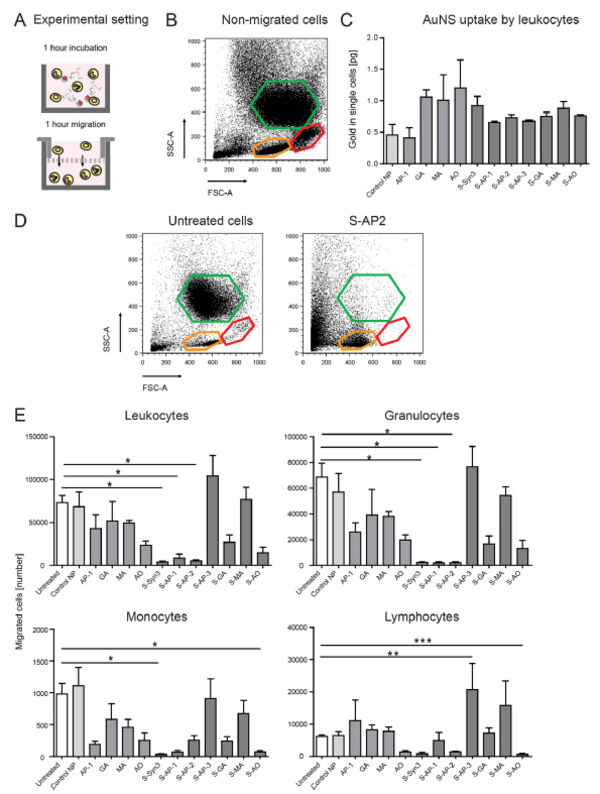
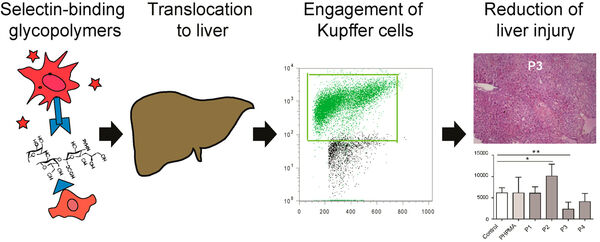
Follow-up studies on carbohydrate-based selectin binding nanoparticles have identified carbohydrate-based groups that are capable to significantly affect the migration of leukocytes and macrophages (Figure 9)
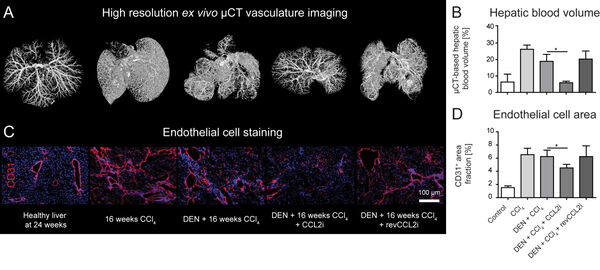
In 2019, we also demonstrated that the inhibition of the monocyte-derived chemokine CCL2 (MCP1) has a profound effect on the development of the hepatic vasculature during tissue injury.
The publication was published on the cover of the journal:
https://www.sciencedirect.com/science/article/pii/S2352345X19300190:
The study was also referenced in an editorial:
https://www.cmghjournal.org/article/S2352-345X(18)30165-6/fulltext
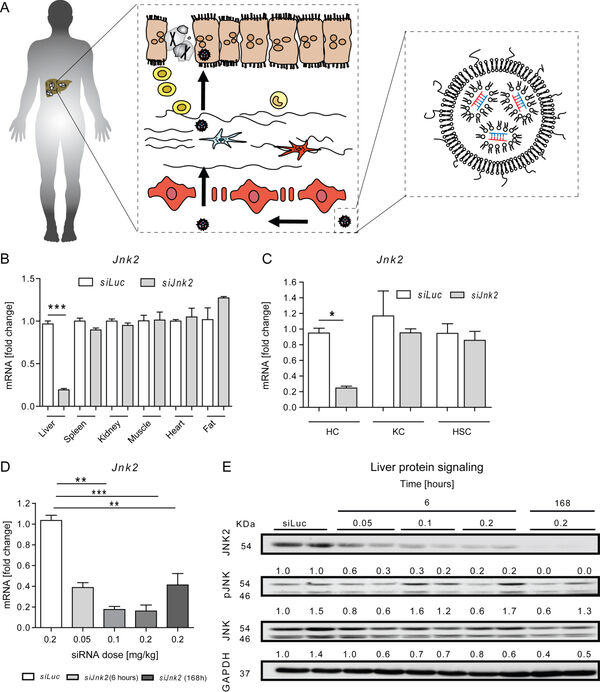
Overnutrition and obesity represent a worldwide burden for the health systems. Furthermore, genetically caused disorders can lead to liver disease and affect other organ systems. Our institute (group of Prof. Trautwein) and the group of Prof. Cubero from Madrid have recently targeted hepatocytes in vivo using a tailored formulation for lipid-based nanocarriers for siRNA against Jnk2 (Figure 11).
4. Cell therapy
Cellular reprogramming of autologous cells of patients is already in the clinics. The lentivral manipulation of T cells to induce tumor cell killing leads to an artificially introduced chimeric antigen receptor to the cells, which are termed CAR T cells. Cells are isolated from the respective patients and are reprogrammed ex vivo. After the genetic modifications, the CAR T cells are injected back into the patient to specifically attack tumor cells.
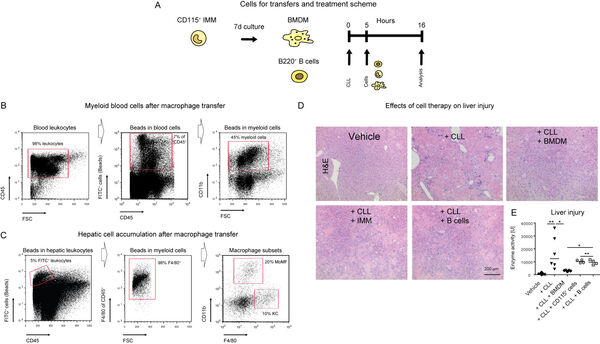
We have recently demonstrated that macrophages exhibit important regulatory functions for genetically induced liver disease (Figure 12A-E). It is therefore concluded that therapies facilitating myeloid cells might represent a feasible strategy for treating inflammatory liver disease.
References
- Bartneck M, Heffels KH, Pan Y, Bovi M, Zwadlo-Klarwasser G, Groll J. Inducing healing-like human primary macrophage phenotypes by 3D hydrogel coated nanofibres. Biomaterials33, 4136-4146 (2012).
- Bartneck M, Skazik C, Paul NE, Salber J, Klee D, Zwadlo-Klarwasser G. The RGD coupling strategy determines the inflammatory response of human primary macrophages in vitro and angiogenesis in vivo. Macromol Biosci14, 411-418 (2014).
- Bartneck M, et al. Molecular response of liver sinusoidal endothelial cells on hydrogels. Mater Sci Eng C Mater Biol Appl51, 64-72 (2015).
- Bartneck M, et al. Histidine-rich glycoprotein promotes macrophage activation and inflammation in chronic liver disease. Hepatology63, 1310-1324 (2016).
- Kreimendahl F, et al. Macrophages significantly enhance wound healing in a vascularized skin model. Journal of biomedical materials research Part A107, 1340-1350 (2019).
- Bartneck M, et al. Fluorescent cell-traceable dexamethasone-loaded liposomes for the treatment of inflammatory liver diseases. Biomaterials37, 367-382 (2015).
- Bartneck M, et al. Immunomodulatory Therapy of Inflammatory Liver Disease Using Selectin-Binding Glycopolymers. ACS Nano11, 9689-9700 (2017).
- Tavernaro I, et al. Modulating Myeloid Immune Cell Migration Using Multivalently Presented Monosaccharide Ligands for Advanced Immunotherapy. Advanced Therapeutics2, 1900145 (2019).
- Bartneck M, et al. The CCR2(+) Macrophage Subset Promotes Pathogenic Angiogenesis for Tumor Vascularization in Fibrotic Livers. Cell Mol Gastroenterol Hepatol7, 371-390 (2019).
- Woitok MM, et al. Lipid-encapsulated siRNA for hepatocyte-directed treatment of advanced liver disease. Cell death & disease11, 343 (2020).
- Bartneck M, et al. Roles of CCR2 and CCR5 for hepatic macrophage polarization in mice with liver parenchymal cell-specific NEMO deletion. Cell Mol Gastroenterol Hepatol, (2020).
Recent book chapters
- Book chapter “Nanomedicines in immunotherapy: limitations and capabilities”, Bartneck M. in the book Current Issues in Nanomedicine, volume 5. 2020
- Book chapter “Nanotechnology in Cancer Theranostics” in the book “Advances in Cancer Nanotheranostics”. Mostafa A. and Bartneck M., 2020, 47-82.
- Therapeutic Targeting of Neutrophil Granulocytes in Inflammatory Liver Disease. Bartneck M, Wang J. Front Immunol. Front Immunol. 2019; 10: 2257.
Editorial activities
- Editorial board member of the nature journal scientific reports
https://www.nature.com/srep/about/editorial-board - Associate Editor of the journal Advances in Polymer technology
https://www.hindawi.com/journals/apt/editors/ - Associate Editor of the journal Annals in Translational Medicine
http://atm.amegroups.com/about/editorialTeam