Schwerpunkte der Arbeitsgruppe / Research focus
Our group is interested in apoptosis and cell cycle regulation in the liver. Key proteins involved in these two basic mechanisms are investigated in the context of chronic liver disease and hepatocarcinogenesis (Fig. 1).
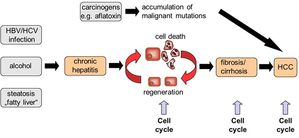
Fig. 1: Several stimuli such as viral infection, alcohol abuse or excessive food intake may result in chronic hepatitis and subsequent liver cell death. As a response to liver injury, healthy liver cells such as hepatocytes and hepatic stellate cells can undergo one or several rounds of cell division in order to repair the damaged liver tissue. However, uncontrolled cell cycle activity will result in liver fibrosis or hepatocellular carcinoma (HCC).
Currently, our lab is focused on four different projects as specified below:
I. The role of E-Cyclins for liver regeneration and hepatocarcinogenesis
The mammalian cell cycle is predominantly controlled by cyclin-dependent kinases and their regulating subunits termed cyclins. Each phase of the cell cycle (G1: Gap-1 phase, S: DNA-synthesis; G2: Gap-2 phase; M: mitosis) is regulated by a kinase complex of a specific cyclin with its cognate Cdk (Fig. 2). The majority of mammalian cells in vivo are in an inactive (quiescent) phase of the cell cycle termed G0. The cell cycle machinery is usually activated by the presence of mitogen factors leading to induction of the D-type cyclins (CcnD1, CcnD2, CcnD3) which bind to and activate Cdk4/Cdk6, leading to phosphorylation and thus inactivation of the retinoblastoma protein (Rb). Rb phosphorylation is completed by CcnE/Cdk2 kinase complexes shortly before entry into S-phase eventually leading to activation of E2F transcription factors and induction of cell cycle related genes. Accordingly, it is believed that CcnE/Cdk2 activity is essential for the initiation of DNA replication from quiescence. After initiation of DNA synthesis, Cdk2 interacts with CcnA and triggers S-phase progression. At the end of interphase, CcnA activates Cdk1 to facilitate the onset of mitosis. After nuclear envelope breakdown, A-type cyclins are degraded, enabling the formation of CcnB-Cdk1 complexes. B-type cyclins in association with Cdk1 are essential for triggering mitosis.
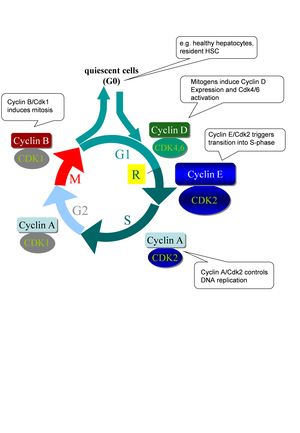
Fig. 2: Canonical model of cell cycle regulation in mammals. G1: Gap-1 phase, S: DNA-synthesis; G2: Gap-2 phase; M: mitosis; R: restriction point. After passing this state cells do no longer depend on mitogens for further progression through the cell cycle. Further explanations are given in the text.
However, this simplified view gets complicated by the fact that mammals typically express three different D-type and two different E, A and B-type cyclins, respectively. Surprisingly, most of these cyclins and their associated Cdks are dispensable for general cell cycle during development or in isolated murine embryonic fibroblasts.
Liver regeneration following partial (70%) hepatectomy (PH) in mice is one of the best experimental models for analyzing cell cycle regulation in vivo. Due to the high regenerative capacity of the liver, resting remnant hepatocytes leave their quiescent state and undergo 1-2 synchronized rounds of cell cycle with a peak of DNA synthesis after approximately 40-48 h resulting in restoration of original liver mass within 7-10 days.
We have recently demonstrated that CcnE1 and CcnE2 have unique functions after PH and even play antagonistic roles. CcnE2-deficient livers showed accelerated and sustained DNA synthesis and hepatomegaly, whereas ablation of CcnE1 provoked only a minor delay of hepatocyte proliferation. In a very recent study we aimed to define the relevance of all Cdk2/CcnE complex components (Cdk2, CcnE1 and CcnE2) for DNA synthesis and cell cycle re-entry in vivo. We generated viable mouse mutants harbouring all possible combinations of CcnE1/CcnE2 and Cdk2 knockout alleles and studied the consequences for hepatocyte proliferation in vitro and in vivo. We demonstrated that CcnE1 – but not its homologue CcnE2 - is essential for driving G0/S-phase transition in hepatocytes lacking Cdk2 and identify minimal genetic requirements concerning Cdk2 and E-cyclins to drive the hepatic cell cycle. Currently, we are investigating the role of E-type cyclins and Cdk2 for the initiation and progression of hepatocellular carcinoma (HCC).
Responsible scientist: M.Sc. Roland Sonntag
II. Cell Cycle regulation during initiation and progression of liver fibrosis
Liver fibrosis is a chronic wound healing process leading to liver scaring by excessive accumulation of extracellular matrix proteins including collagen. The main producers of liver collagen are myofibroblasts that develop from activated hepatic stellate cells (HSC). Additionally, other cell types such as portal fibroblasts and bone marrow derived cells may contribute to extracellular matrix production. Liver fibrosis develops on the basis of chronic liver injury induced e.g. by chronic viral hepatitis and steatohepatitis. Although these basic mechanisms of liver fibrogenesis are well understood, it has been widely ignored so far that initiation of liver fibrosis requires cell cycle activation of at least HSC and remnant hepatocytes as a response to liver injury and subsequent cell loss. Transition of quiescent cells into the proliferative phase of the cell cycle is controlled by E-type cyclins (CcnE1, CcnE2) and the associated cyclin-dependent kinase 2 (Cdk2). Therefore, the overall working hypothesis of our project is that CcnE-dependent activation of the cell cycle in hepatic cell populations is a prerequisite for liver fibrogenesis. We recently demonstrated that CcnE1 – but not its homologue CcnE2 – is up-regulated in patients and in mice with advanced liver fibrosis. Using CcnE1 knockout mice, we provided evidence that CcnE1 is essential for cell cycle progression and survival of HSC in vitro and for the onset of liver fibrosis in vivo defining CcnE1 and/or Cdk2 as potential attractive targets for pharmacological intervention in patients with chronic liver disease. Currently, we are investigating the underlying mechanisms in more detail and aim to translate these findings into therapeutic strategies.
Responsible scientist: Dr. Jörg-Martin Bangen
III. The impact of alcohol intake for cell cycle control in the liver
Hepatocellular carcinoma (HCC) is one of the most frequent primary tumors with growing incidence and poor prognosis. One of the proven risk factors for the development of HCC is chronic alcohol abuse. Although it is obvious that HCC initiation and progression is associated with excessive proliferation of hepatocytes, the mechanisms of alcohol-mediated initiation and progression of liver disease is still poorly understood. This is in part due to the unexpected and contradictory observation that alcohol primarily inhibits hepatocyte proliferation and liver regeneration. In this project, which is supervised by Dr. Yulia Nevzorova, we aim to clarify the mechanisms resulting in HCC due to chronic alcohol consumption using appropriate mouse models. With this approach we hope to identify novel target genes allowing a better therapy of alcoholic liver disease and its consequences.
Responsible scientist: Dr. Yulia Nevzorova
IV. The role of the pro-apoptotic mediator caspase-8 for acute and chronic liver disease
The cysteine-aspartate protease caspase-8 has a central function as the initiator caspase in extrinsic apoptotic signaling pathways. With respect to the liver, several studies suggested that caspase-8 might be relevant for acute liver failure, but also for some forms of chronic liver disease and hepatocarcinogenesis.
In recent years we have developed genetically modified mice allowing conditional inactivation of caspase-8 in different cell types with the ultimate aim to test, if caspase-8 inhibition could be a treatment option for patients with liver disease.
Surprisingly our studies so far revealed that inhibition of caspase-8 mediates either protective or harmful effects depending on the tissue environment and the involved cell type. For instance, caspase-8 inhibition in hepatocytes is protective in models of Fas-mediated acute liver injury and during steatohepatitis, while it induces a novel form of severe liver necrosis termed necroptosis in an inflammatory environment. Currently, we aim to better define the molecular pathways that define if caspase-8 inhibition drives non-apoptotic cell death or survival signals.
Responsible scientist: B.Sc. Nadine Hoeltke



