Translational Neurophysiology & Neural Disease Modeling in the Human Brainscape
Translational neurophysiology is dedicated to investigating physiological and pathological processes in the human brain with the goal of transforming these insights into improved diagnosis, treatment, and prevention of neurological diseases. A key element of this work is the use of human experimental systems, as animal models—despite their scientific value—often face limitations in their translational relevance.
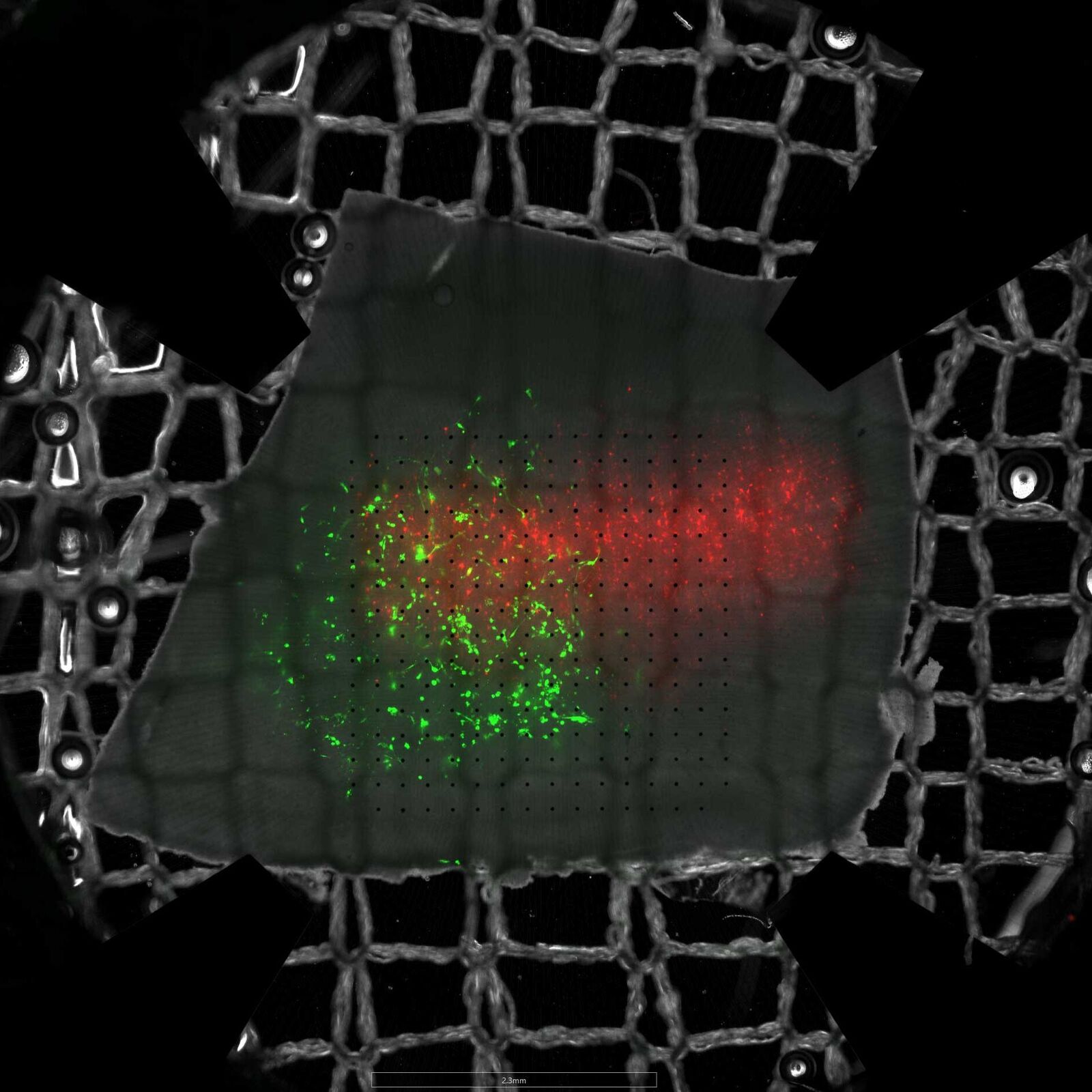
Our research focuses on organotypic brain slice cultures derived from human tissue, which allow for the study of neuronal networks in both healthy and diseased states under conditions that closely mimic the in vivo environment. This model offers unique insights into disease-specific mechanisms directly within the human context and provides a promising platform for the development and testing of therapeutic strategies.
Our overarching aim is to bridge the gap between experimental neuroscience and clinical application—by employing reductionist yet human-relevant model systems that are ethically responsible and pave the way for forward-looking, human-centered research.

Aniella Bak
abakukaachende
Address
Dept. of Neurology, Section Epileptology
University of Aachen
Pauwelsstrasse 30
52074 Aachen, Germany
Tel. +49-(0)80-338786

Birgit Gittel (lead technician)
bgittelukaachende
Address
Dept. of Neurology, Section Epileptology
University of Aachen
Pauwelsstrasse 30
52074 Aachen, Germany
Tel. +49-(0)241-80-38611

Carina Haaken
chaakenukaachende
Address
Dept. of Neurology, Section Epileptology
University of Aachen
Pauwelsstrasse 30
52074 Aachen, Germany
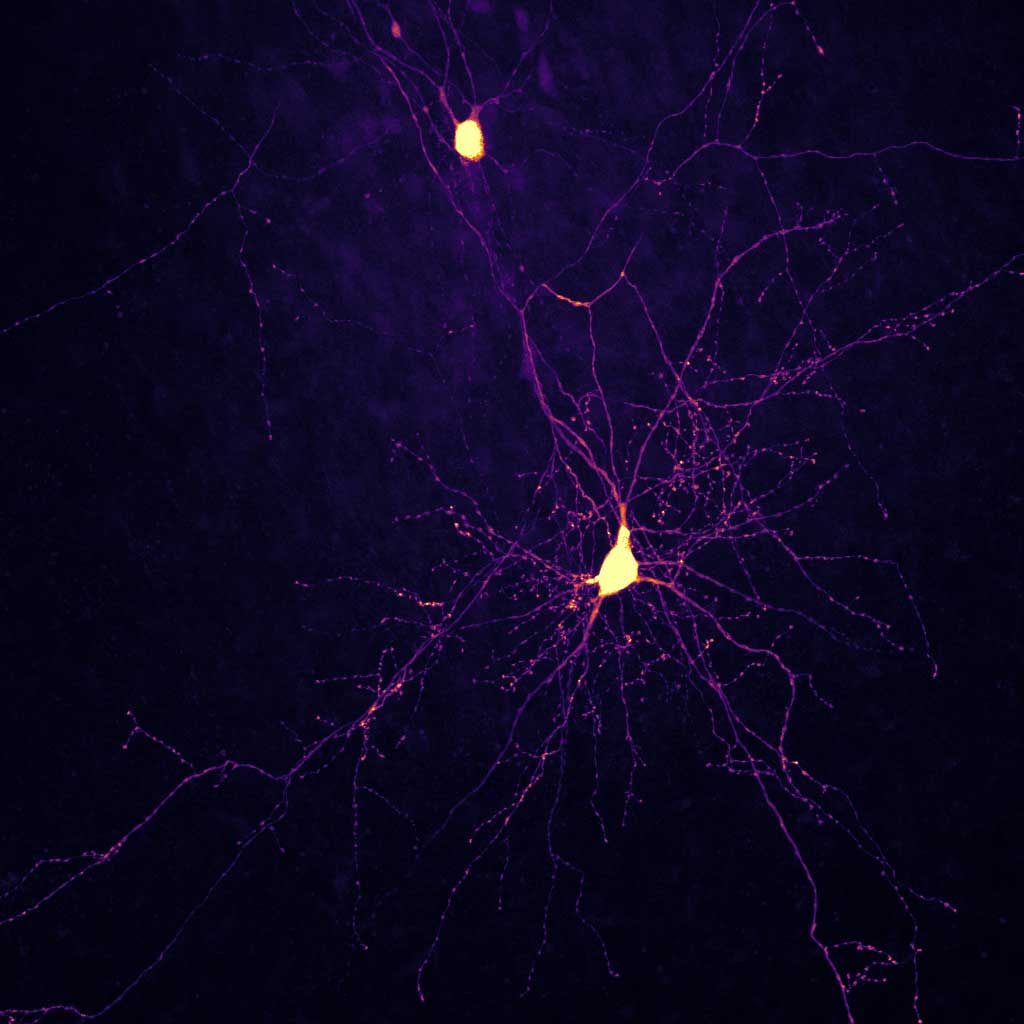
Organotypic brain slice cultures can be prepared from mouse or human brain tissue and preserve the three-dimensional architecture of the original brain region, including its native cell types, their interactions, and the genetic and molecular background of the tissue donor. These cultures can be maintained in vitro for several weeks, providing a unique opportunity for long-term observation, manipulation, and analysis. Human brain tissue is particularly delicate, which is why we continuously work to optimize our protocols to enhance the viability and quality of our cultures. In parallel, we systematically characterize how the tissue adapts, responds, and evolves over time in vitro.
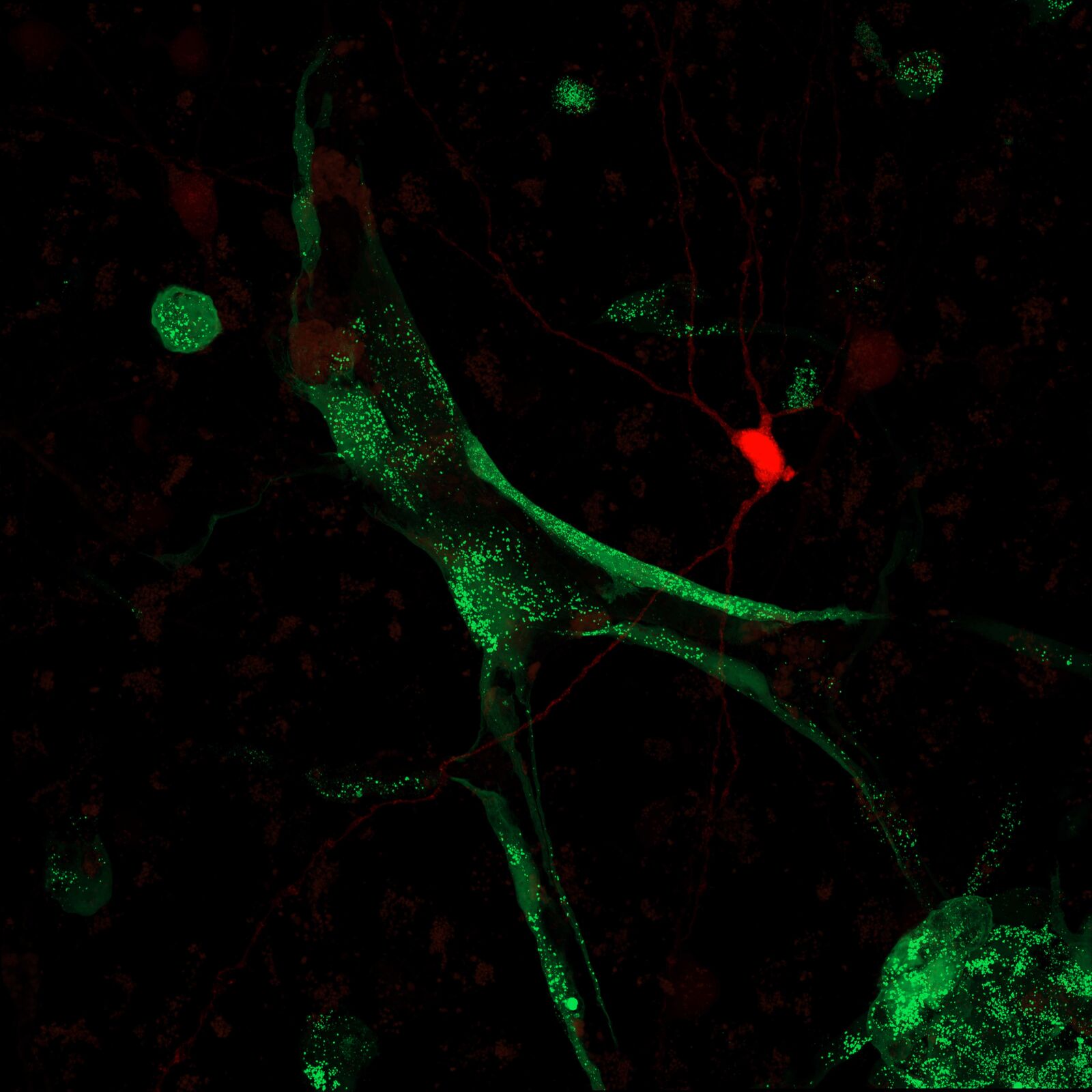
Glioblastoma is an aggressive and devastating brain tumor with an extremely poor patient prognosis. Although numerous studies on its pathogenesis, progression, and treatment have been conducted in animal models, research in human tissue is essential to enable meaningful translation to the human system. We have established a co-culture model of primary patient-derived glioblastoma cells and human cortical tissue, allowing us to investigate the interaction between tumor cells and the endogenous human neuronal network on electrophysiological, molecular, and transcriptomic levels..
Epilepsy is a chronic neurological disorder characterized by recurrent, unprovoked seizures and affects approximately 50 million people worldwide. It can have various causes, including genetic mutations, brain injuries, developmental disorders, and infections. Mutations in genes not previously associated with epilepsy—referred to as “epilepsy candidate genes”—are increasingly identified as potential contributors to the disease. In our brain slice cultures, we can model both genetic epilepsies, for example, by knocking down an epilepsy candidate gene, and acquired epilepsies, such as those resulting from brain injury. This system allows us to investigate the process of epileptogenesis, characterize the resulting phenotype and underlying molecular dysfunctions, and screen for potential new therapeutic strategies.
Yang, D., Qi, G., Ort, J., Witzig, V., Bak, A., Delev, D., Koch, H., & Feldmeyer, D. (2024). Modulation of large rhythmic depolarizations in human large basket cells by norepinephrine and acetylcholine. Communications Biology, 7, 885. https://doi.org/10.1038/s42003-024-06546-2
Bak, A., Koch, H., van Loo, K. M. J., Schmied, K., Gittel, B., Weber, Y., Ort, J., Schwarz, N., Tauber, S. C., Wuttke, T. V., & Delev, D. (2023). Human organotypic brain slice cultures: A detailed and improved protocol for preparation and long-term maintenance. Journal of Neuroscience Methods, 404, 110055. https://doi.org/10.1016/j.jneumeth.2023.110055
This paper was honored with the “Paper of the Quarter” award for the first quarter of 2024 from the 3R competence network NRW.
Bak, A., Schmied, K., Jakob, M. L., Bedogni, F., Squire, O. A., Gittel, B., Jesinghausen, M., Schünemann, K. D., Weber, Y., Kampa, B., van Loo, K. M. J., & Koch, H. (2024). Temporal dynamics of neocortical development in organotypic mouse brain cultures: A comprehensive analysis. Journal of Neurophysiology, 132(3). https://doi.org/10.1152/jn.00178.2024
Qi, G., Yang, D., Bak, A., Hucko, W., Delev, D., Hamou, H., Feldmeyer, D., & Koch, H. (2024). Transport-related effects on intrinsic and synaptic properties of human cortical neurons: A comparative study. bioRxiv. https://doi.org/10.1101/2024.10.30.621044
Schünemann, K. D., Hattingh, R. M., Verhoog, M. B., Yang, D., Bak, A. V., Peter, S., van Loo, K. M. J., Wolking, S., Kronenberg-Versteeg, D., Weber, Y., Schwarz, N., Raimondo, J. V., Melvill, R., Tromp, S. A., Butler, J. T., Höllig, A., Delev, D., Wuttke, T. V., Kampa, B. M., & Koch, H. (2025). Comprehensive analysis of human dendritic spine morphology and density. Journal of Neurophysiology, 133(4), 1086–1102. https://doi.org/10.1152/jn.00622.2024
Jütten, K., Ort, J., Kernbach, J. M., Meyer-Baese, A., Meyer-Baese, U., Hamou, H. A., Clusmann, H., Wiesmann, M., Bremer, J., Koch, H., Bak, A., Ricklefs, F., Drexler, R., Heiland, D.-H., & Delev, D. (2025). High peritumoral network connectedness in glioblastoma reveals a distinct epigenetic signature and is associated with decreased overall survival. Neuro-Oncology. Advance online publication. https://doi.org/10.1093/neuonc/noaf101
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/files/klinik-neurologie/_processed_/5/6/csm_Header_Neurologie_Farbverlauf8_3df2c3e92c.jpg)
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/files/klinik-neurologie/_processed_/5/6/csm_Header_Neurologie_Farbverlauf9_9b298c84b0.jpg)
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/files/klinik-neurologie/_processed_/1/7/csm_Header_Neurologie_Farbverlauf10_f2a6557cd2.jpg)
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/files/klinik-neurologie/_processed_/8/3/csm_Header_Neurologie_Farbverlauf11_13492b1a7c.jpg)
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/files/klinik-neurologie/_processed_/c/6/csm__ME11795_a09a0eccd2.jpg)
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/files/klinik-neurologie/_processed_/0/d/csm_Header_Neurologie_Farbverlauf12_259bdd652b.jpg)