Molecular Dynamics Guided Analysis of Ligand Activation, Ion Permeation and Subtype-specific Ligand Interactions in P2X Receptors
Principal Investigator: Prof. Dr. med. Ralf Hausmann
Co-PI: Jun.-Prof. Dr. med. Jan-Philipp Machtens (Institute of Clinical Pharmacology of RWTH Aachen University and Computational Neurophysiology (ICS-4) of Forschungszentrum Jülich)
P2X receptors are a family (P2X1R–P2X7R) of trimeric ligand-gated ion channels that mediate the depolarizing influx of cations into cells upon binding of extracellular ATP and are interesting targets for new drugs. In the last years, significant advances in our understanding of the molecular function, modulation, and structure of P2XRs have been made.
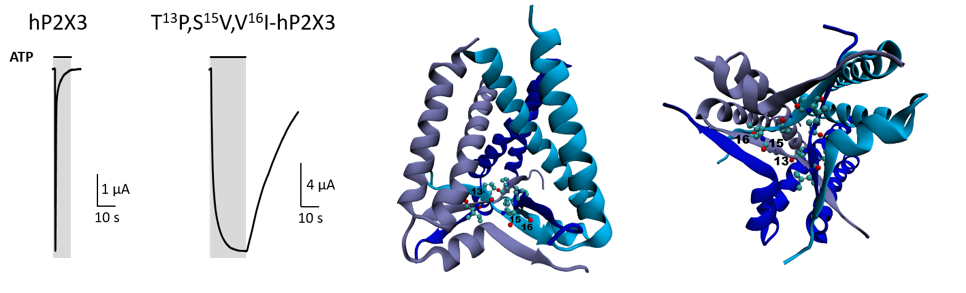
Our former studies have identified three N-terminal, intracellular amino acid residues that markedly affect the desensitization kinetics of the P2X3R (Hausmann et al., 2014). The T13P/S15V/V16I mutation virtually abolished desensitization of the P2X3R (Fig. 1) and was indispensable for capturing the ATP-bound open-state X-ray crystal structure of the hP2X3R (Mansoor et al., 2016).
The release of the first high-resolution X-ray crystal structures of mammalian P2XRs, the human P2X3R (Mansoor et al., 2016) are crucial for the project. The open-, closed-, and desensitized-state hP2X3R structures provide an unprecedented opportunity to reliably bridge these structures with functional measurements via extensive all-atom molecular dynamics (MD) simulations (Fig. 2).
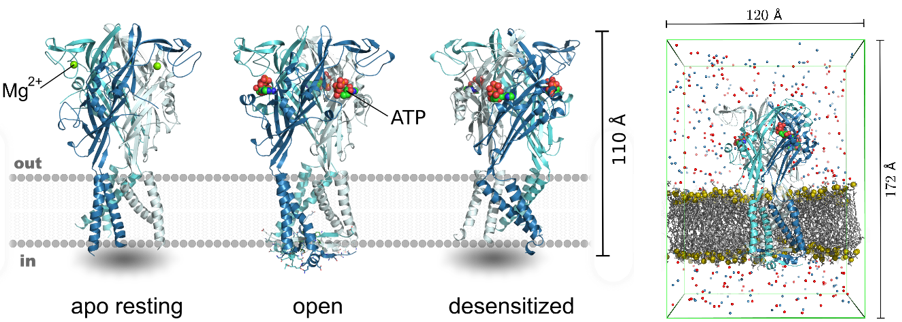
The main objectives of the project are to identify the dynamic molecular mechanisms of ligand activation and gating, along with the molecular mechanisms responsible for ion permeation, conductance, and selectivity. A related objective is to identify the molecular determinants of subtype-specific ligand interactions to understand how P2XRs can be selectively targeted by drugs. We comprehensively use MD simulations, ligand-docking, and virtual screening simulations along with site-directed mutagenesis and electrophysiological analysis at the macroscopic and single channel levels to understanding the functional dynamics of ligand binding-coupled ion flow in P2XRs in atomic detail.
Mansoor et al., 2016. X-ray structures define human P2X3 receptor gating cycle and antagonist action. Nature 2016, 538:66-71.
Hausmann et al., 2014. A hydrophobic residue in position 15 of the rP2X3 receptor slows desensitization and reveals properties beneficial for pharmacological analysis and high-throughput screening. Neuropharmacology 2014, 79:603-15.








