Christian Hasberg MSc

Structure-function analysis of the plasma protein fetuin-A
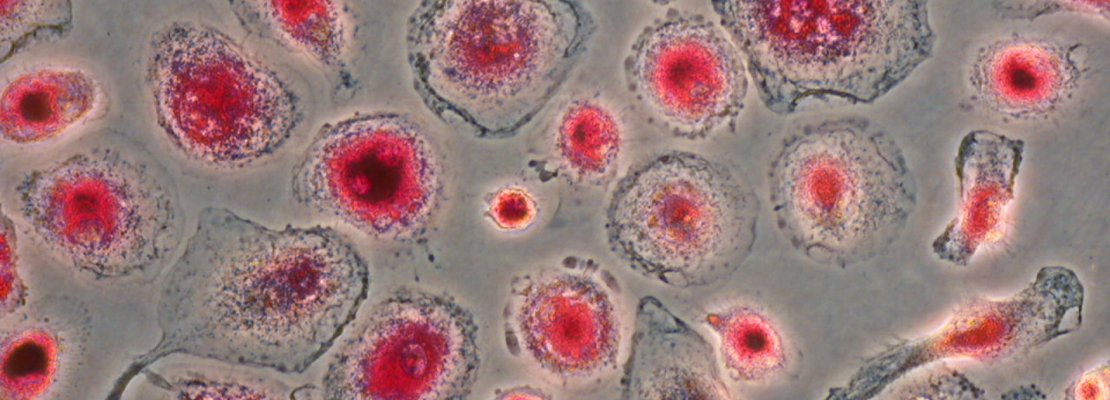
Mineral crystals are incorporated into the matrices of living creatures through the controlled process of biomineralization, creating a solid framework for the remaining body components. As calcification is physiologically limited to the collagenous matrix of bones and teeth, a variety of local and circulatory calcification inhibitors actively prevent this in soft tissues. Fetuin-A, a plasma protein produced from the liver, controls mineralization. Fetuin-A is a systemic inhibitor of ectopic calcification. By generating colloidal protein-mineral complexes, fetuin-A stabilizes saturated mineral solutions. The binding of minerals at the amino acid level is mediated by certain functional groups, such as phosphorylation sites.
We produce point-mutated Fetuin-A variants in our lab and analyze them regarding their altered functionality to determine which functional groups affect the binding of calcium phosphates or other minerals. The 3D structure of the protein shall be determined by cryo-EM alongside to functionality tests in order to define the interaction between the protein and the mineral facilitating biomineralization.