Contact

Director
Univ.-Prof. Dr. rer. nat. Oliver Pabst
Phone: +49 241 80-85496
opabstukaachende
AG Pabst (ukaachen.de)

Office
Betül Asik
Phone: +49 241 80-85686
Fax: +49 241 80-82094
baycicekukaachende
Level 5, Corridor D, Room 20
Doctoral candidates

Therese Hubert
Therese Hubert
thubertukaachende
My project:
The adaptive immune response is orchestrated by a diverse array of immune cell subsets, each with specialized roles. While most of these cells, such as CD8+ T cells, are primed to detect and eliminate foreign antigens, a distinct subset—T regulatory cells (Tregs)—plays a crucial role in maintaining immune homeostasis and preventing autoimmune reactions. Interestingly, tumors have evolved mechanisms to exploit Tregs, using their immunosuppressive function to promote tumor survival and growth. In mouse models of intestinal cancer, for example, an accumulation of Tregs is observed in response to early tumoral changes within the gut. However, the precise mechanisms by which Tregs exert their immunosuppressive effects—particularly how they inhibit the cytotoxic activity of CD8+ T cells—remain poorly understood. The goal of my project is to investigate the interactions between Tregs and CD8+ T cells in the context of intestinal tumors and their role in metastasis. By identifying and characterizing the molecules that mediate these interactions, we hope to uncover new insights that could inform future immunotherapeutic strategies for cancer treatment.
My project:
The leading cause of mortality in colorectal cancer (CRC) is liver metastasis. Both the gut and liver are highly tolerogenic organs that require tight immune regulation to prevent inflammation. Immune cells, such as regulatory T cells (Tregs), play a key role in maintaining this balance. However, in cancer, Tregs can promote immune evasion. Understanding the immune environments of the gut and liver during tumor progression is crucial for improving diagnosis and developing targeted therapies. Using a mouse model that mimics early tumor progression, we observed an accumulation of KLRG1+ Tregs in the gut. This model provides a unique platform to investigate how gut immune cells influence the liver during early tumor development. Through high-dimensional spectral flow cytometry, NGS TCR sequencing, multiplex immunostaining, and other tumor mouse models, my project aims to uncover key immune pathways linking the gut and liver during neoplasia, ultimately contributing to more effective therapeutic strategies targeting immune regulation.
My project:
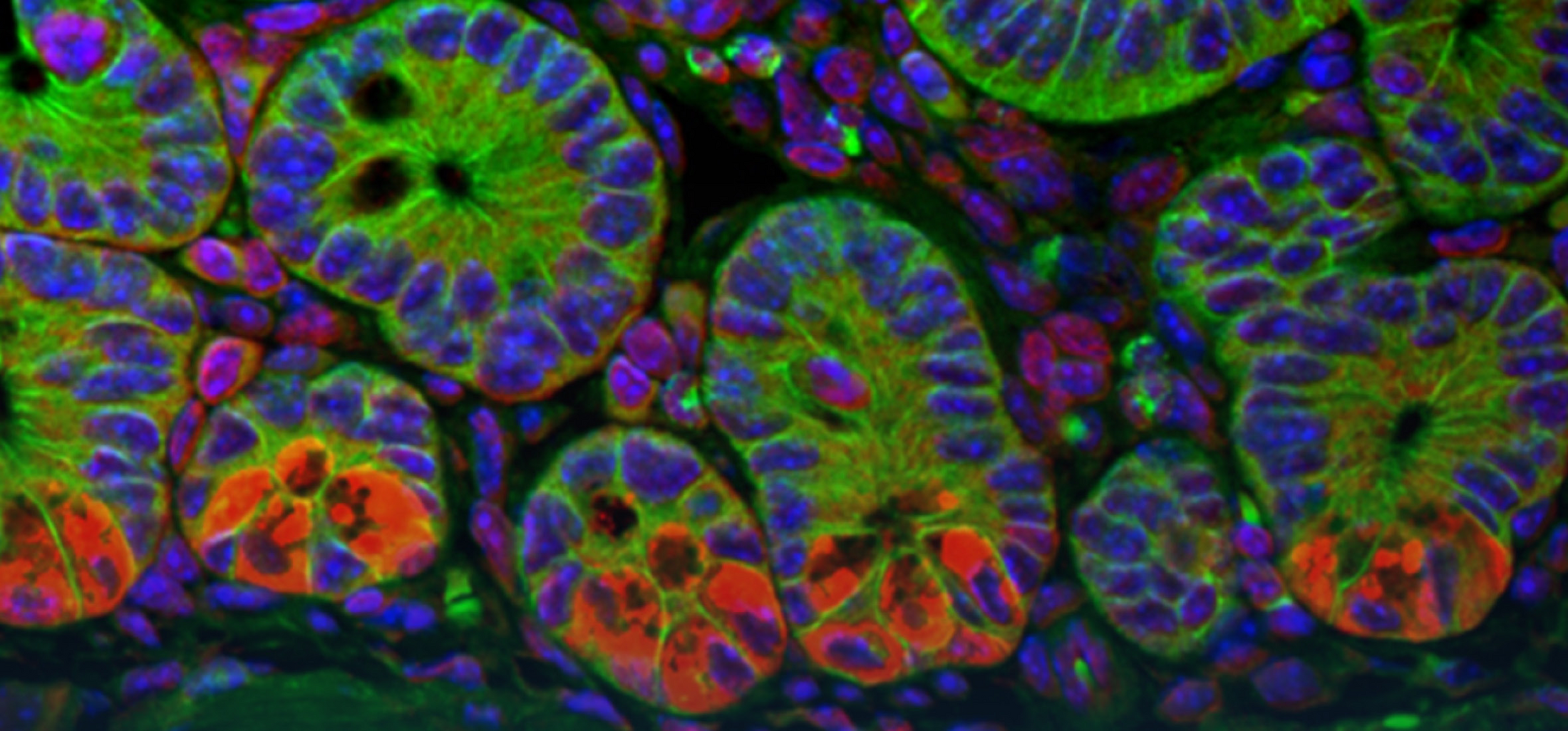
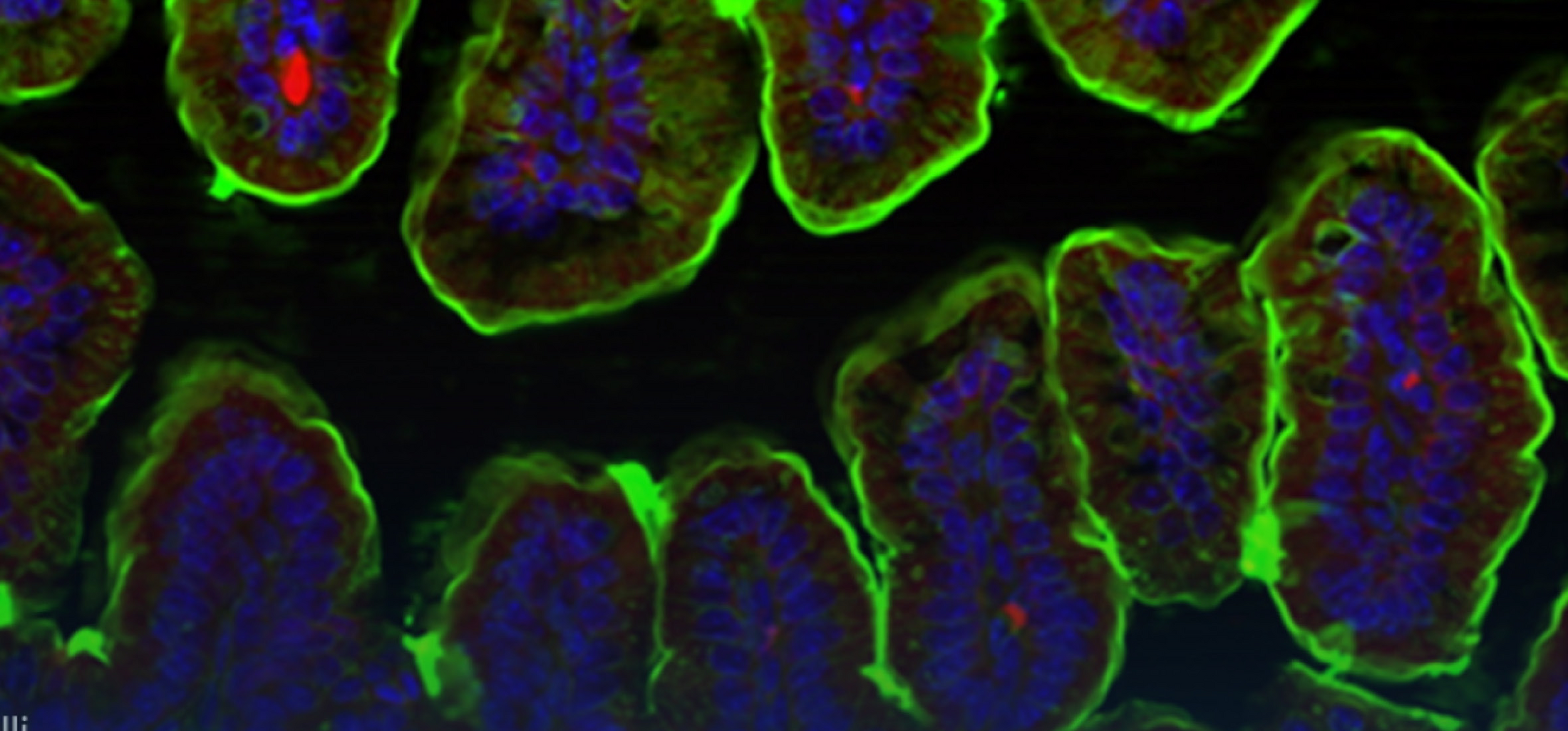
Immunoglobulin A (IgA) is a key host factor that regulates the composition and distribution of the gut microbiota and the defence against enteropathogens. IgA is produced by plasma cells in the intestinal lamina propria and is a fundamental element in organising a complex network of interactions between the intestinal epithelium, the microbiota and the immune system. However, despite its importance, the effects of IgA on the integrity and function of the intestinal barrier are poorly understood. Therefore, my aim is to understand the function of IgA on intestinal epithelial cells and the intestinal barrier. To this end, I am investigating epithelial cell function and phenotype in the absence of IgA, as well as the spatio-phenotypic characteristics of plasma cells using a newly established AI-based image analysis pipeline. We anticipate that this knowledge will be critical in developing a comprehensive understanding of the gut barrier and it's defects in human disease.

Alvaro Panadero
Alvaro Panadero
apanaderoukaachende
My project:
Immunoglobulin A nephropathy (IgAN) is the most common cause of primary glomerulonephritis worldwide, with up to 30% of cases progressing to kidney failure within 10 years after diagnosis. In IgAN, glomerular injury is initiated by the binding of immune deposits containing galactose-deficient IgA1 (Gd-IgA1) to human mesangial cells, ultimately leading to kidney damage. However, many questions remain unanswered, including the underlying causes of Gd-IgA1, the role of the intestinal immune system, or the pathophysiological consequences of Gd-IgA1 deposition in the kidney. My aim is to better understand the origin, function and pathological impact of poorly galactosylated IgA1 in the disease.
My project:
In humans, approximately 5 g of immunoglobulin A is produced per day, making IgA the most abundant antibody at all. IgA coats commensal bacteria in the gut and affects their growth, motility, metabolism, bile acid sensitivity and interactions with the host. Surprisingly, however, we know little about the actual targets of IgA at the molecular level. I am developing a systematic approach to better define IgA targets in the intestinal immune system. I combine FACS-based enrichment of transposon mutant libraries stained with IgA and high content identification of transposon insertion sites. We expect that a deeper understanding of IgA targets will ultimately be an important step towards therapeutic manipulation of the gut microbiota.
Medical technicians

Shery Ayoub
Shery Ayoub
Phone: 0241 80-85783
sayoubukaachende

Kristina Vukovic
Kristina Vukovic
Phone: +49 241 80-85783
kvukovicukaachende