Molecular biology of signalling in inflammatory processes
Our focus is the investigation of the cell-surface localised protease ADAM17 and its crucial interactor iRhom. The proteolytic activity of the ADAM17-iRhom complex represents a key hub for a variety of important signalling pathways.
Intercellular communication is the foundation of many crucial processes in multicellular organisms. In this regard, growth factors and cytokines and their associated receptors present two of the main intercellular communication routes. Growth factors are involved in triggering fundamental processes like cell proliferation and differentiation. Therefore, they are essential components of development and regeneration. Cytokine signalling is primarily linked to the immune system and inflammatory processes. Consequently, dysfunctions in these signalling pathways cause serious defects such as chronic inflammation, insufficient immunity or cancer. As proper functioning of these signalling systems is of vital significance, the underlying regulation, which balances these systems, is of compelling interest.
A crucial factor of many signalling pathways is the release of soluble, biologically active ectodomains by proteolytic processing of membrane-bound precursors from the cell surface. This process is called ectodomain shedding and involved proteases are often referred to as sheddases. Proteolytically released ectodomains can be either receptor ligands or soluble receptors and therefore can have either agonistic or antagonistic signalling activities. The transmembrane sheddase a disintegrin and metalloproteinase (ADAM) 17 sheds various substrates and by this acts as a key regulator in many crucial physiological processes. Its substrates include epidermal growth factor receptor (EGFR) ligands as well as different cytokines such as the tumour necrosis factor (TNF)-α. This variety of substrates requires a tight regulation of the shedding activity. In the past years, many different layers of regulatory processes were discovered which keep ADAM17 in check.
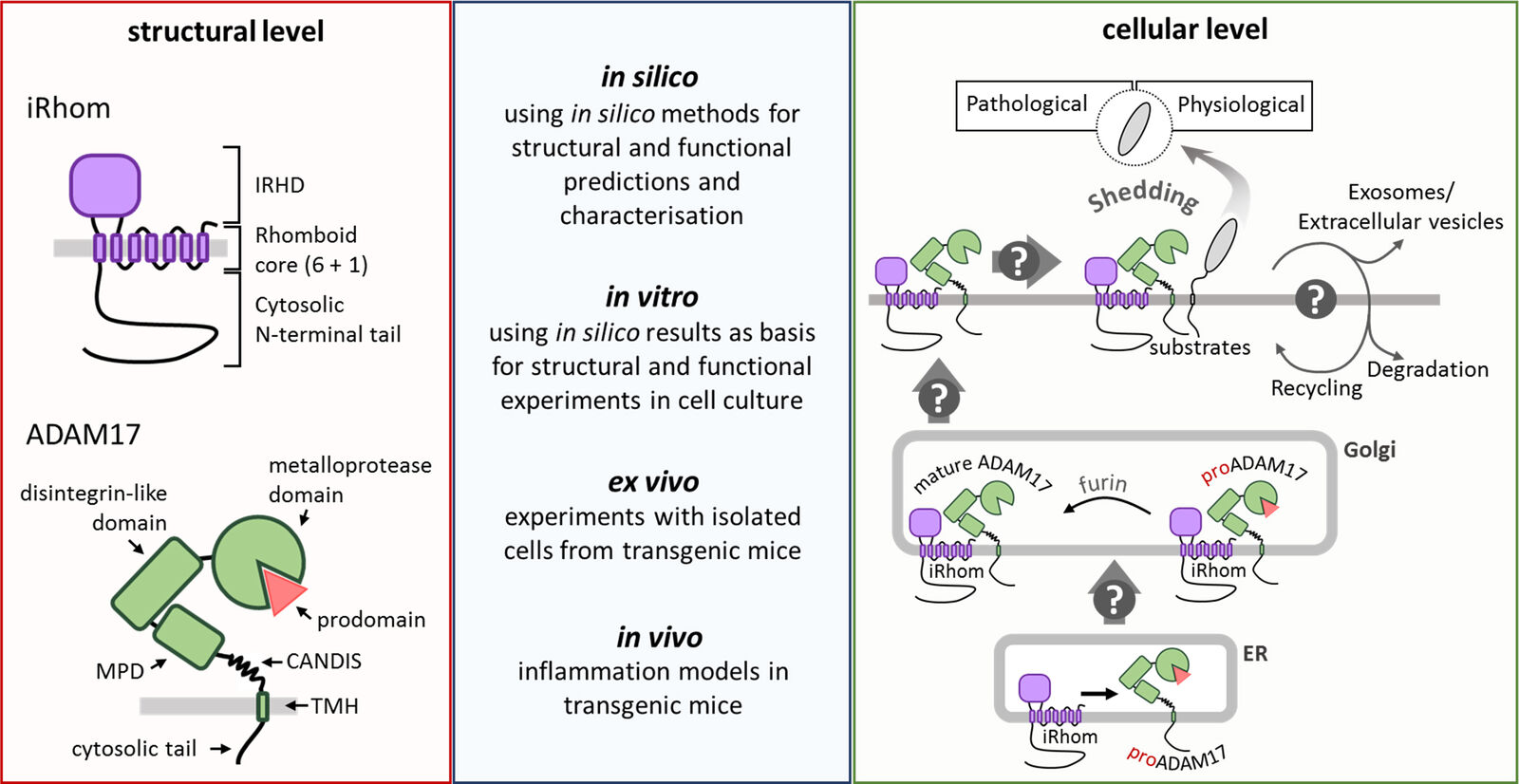
The multi-transmembrane pseudoproteases iRhoms, members of the rhomboid superfamily, have been identified as the most indispensable regulators of ADAM17. They play two important but separated roles for the ADAM17 activity. First, the interaction of ADAM17 with iRhoms is required for the forward trafficking from the ER to the Golgi, where ADAM17 is converted from zymogen to its mature form by furin-like pro-protein convertases. Secondly, iRhoms are directly involved in the regulation of the ADAM17-mediated shedding process on the cell surface.
There are still many open questions regarding the underlying molecular mechanisms of the iRhom-mediated regulation of ADAM17. In our research, we try to characterise the different structural units of iRhoms and thereby try to determine their specific function. To this end, we combine in silico analysis and predictions with different in vitro experiments including divers molecular-biology techniques and cell-biology methods. Furthermore, we use transgenic mice to analyse the iRhom-mediated regulation of ADAM17 in a more physiological setting by performing ex vivo and in vivo experiments.
Career
We are always looking for motivated students for Bachelor's and Master's theses as well as for medical doctoral theses. If you are interested, please contact Dr. Stefan Düsterhöft (sduesterhoeftukaachende).
- Düsterhöft, S., S. Jung, C. W. Hung, A. Tholey, F. D. Sönnichsen, J. Grötzinger and I. Lorenzen (2013). "Membrane-proximal domain of a disintegrin and metalloprotease-17 represents the putative molecular switch of its shedding activity operated by protein-disulfide isomerase." J Am Chem Soc135(15): 5776-5781.
- Düsterhöft, S., K. Höbel, M. Oldefest, J. Lokau, G. H. Waetzig, A. Chalaris, C. Garbers, J. Scheller, S. Rose-John, I. Lorenzen and J. Grötzinger (2014). "A disintegrin and metalloprotease 17 dynamic interaction sequence, the sweet tooth for the human interleukin 6 receptor." J Biol Chem289(23): 16336-16348.
- Düsterhöft, S., M. Michalek, F. Kordowski, M. Oldefest, A. Sommer, J. Röseler, K. Reiss, J. Grötzinger and I. Lorenzen (2015). "Extracellular Juxtamembrane Segment of ADAM17 Interacts with Membranes and Is Essential for Its Shedding Activity." Biochemistry54(38): 5791-5801.
- Sommer, A., F. Kordowski, J. Buch, T. Maretzky, A. Evers, J. Andra, S. Düsterhöft, M. Michalek, I. Lorenzen, P. Somasundaram, A. Tholey, F. D. Sönnichsen, K. Kunzelmann, L. Heinbockel, C. Nehls, T. Gutsmann, J. Grötzinger, S. Bhakdi and K. Reiss (2016). "Phosphatidylserine exposure is required for ADAM17 sheddase function." Nat Commun7: 11523.
- Lorenzen, I., J. Lokau, Y. Korpys, M. Oldefest, C. M. Flynn, U. Künzel, C. Garbers, M. Freeman, J. Grötzinger and S. Düsterhöft (2016). "Control of ADAM17 activity by regulation of its cellular localisation." Sci Rep6: 35067.
- Düsterhöft S., U. Künzel and M. Freeman (2017) "Rhomboid proteases in human disease: mechanisms and future prospects" Biochim Biophys Acta.1864(11 Pt B): 2200-2209.
- Grötzinger J., I. Lorenzen and S. Düsterhöft (2017)" Molecular insights into the multi-layered regulation of ADAM17: The role of the extracellular region" Biochim Biophys Acta.1864(11 Pt B): 2088-2095.
- Düsterhöft S., J. Lokau and C. Garbers(2019) “The metalloprotease ADAM17 in inflammation and cancer" Pathol Res Pract.6:152410.