Computational Simulation of Subcellular Neurobiological Processes

Jun.-Prof. Dr. Jenny Tigerholm
Joint Research Center for Computational Biomedicine
Scientific Center for Neuropathic pain Aachen SCNAACHEN
Roermonder Str. 110, 52072 Aachen
Floor 1, Room 17
Tel.: 0241 99793 214
jtigerholmukaachende
Assistant Prof. Tigerholm has a background in Biophysics and a PhD in computer science from the Royal Institute of Technology in Sweden. She has extensive experience from development of computational models of excitable membranes of the central and peripheral nervous systems. Recently, she has also expanded her lab to include probing peripheral nerves by transcutaneous electrical stimulation. Her current research is focused on understanding pathological alterations in peripheral nerves.
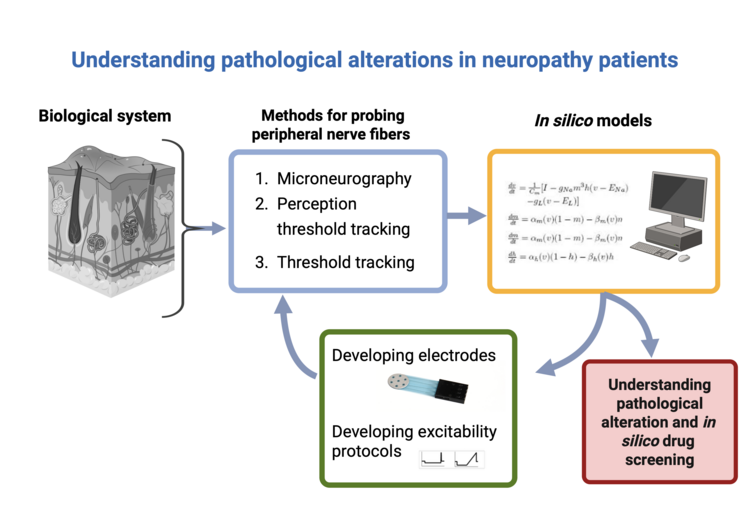
In neuropathy patients, the excitability of peripheral nerves is altered, and these alterations may be a key driver for the underlying pathology. To understand these excitability alterations, Assistant Prof. Tigerholm combines in silico modelling and different experimental setups to probe peripheral nerves. In her lab, they use Microneurography (unmyelinated fibers), Perception threshold tracking (thinly myelinated fibers), and Threshold tracking (large sensory and muscle fibers) to probe the fibers. For each experimental setup, the Tigerholm lab has the corresponding conductance-based multicompartmental model to understand the experimental results as well as to develop the experimental setup further. The computational model combines Hodgkins and Huxley models with finite element models to accurately predict experimental outcomes. The excitability cell membrane models include a wide range of voltage-gated ion channels such as Nav1.6-Nav1.9, HCN, multiple potassium channels, and calcium channels.
The main scientific contributions from the in silico model are:
- Successful development of a novel nerve fiber excitability protocol
- Successful development of a novel electrode for preferential activation of small fibers by cutaneous electrical stimulation
- Explaining microneurography data
The aim is not only to understand pathological peripheral alterations, but also to use in silico drug screening to functionally restore the pathological function.
Identify ion channel dysfunctions in both small and large nerve fibers by utilizing computationally derived excitability tests.
Voltage-gated ion channels play a crucial role in neuropathy due to their essential role in regulating the excitability of peripheral nerves. In this project, we aim to identify pathological alterations in voltage-gated ion channels in humans to understand the underlying pathology. By computational modeling, we derive nerve fiber excitability protocols consisting of electrical pulses that target the unique dynamics of specific subtypes of ion channels. These protocols can be employed using various techniques, such as Threshold Tracking, Perception Threshold Tracking and Microneurography. By combining Hodgkins and Huxley models with the finite element model, we have developed a sodium channel excitability protocol (SCENT). The test consists of five electrical pulse shapes. We used an automated whole-cell patch clamp on Nav1.7 expressed in HEK293T cells and a PTT experiment in healthy participants to validate the SCENT. SCENT provides a non-invasive test to detect Nav1.7 involvement in neuropathic pain patients and may extend to Nav1.8 and Nav1.9, aiding diagnosis and targeted treatment of neuropathic pain.
In the future, we aim to develop excitability protocols for both large and small fibers, as well as for different subtypes of ion channels, including calcium and potassium.

Develop a digital twin of patients with peripheral neuropathic pain to uncover pathological changes and guide personalized treatment.
In this project, we aim to develop a digital twin of the patient's peripheral nerves, which is a virtual representation used for simulation, analysis and optimization. The digital twin will be a digital replica that mirrors the nerve endings, allowing for the identification of pathological alterations, as well as in silico drug screening. The Digital twin will have a representation of four peripheral nerves: C fiber, Aδ fiber, Aβ fibers and muscle fibers. The models will be developed using experimental data from the following experimental setups: Microneurography, Perception Threshold Tracking and Threshold Tracking. In the future, we aim to utilize the digital twin to identify pathological alterations in patients and conduct in silico drug screening once the channelopathy has been identified.


Anna Maxion
Phd student

Alexandra Dzwonkowski
Research assistant

Lea Dworschak
Research assistant

Jennifer Betz
Intern
Project 1: In silico modelling of nociceptors
Background: The Patch-clamp technology has revolutionized our understanding of excitable cell membranes of nociceptors. During a patch-clamp experiment, the membrane potential or the amount of current passing across the membrane can be measured. However, the technique has its limitations. For instance, the reduced temperature during the experiments, compared to the human body, is one of the most considerable limitations. Since the cell membrane excitability is highly sensitive to temperature, this limits the conclusions that can be drawn from patch-clamp experiments. Another limitation is the lack of selective blockers of ion channels, which govern the membrane's excitability. In silico modelling can translate patch-clamp data to physiologically relevant temperatures and evaluate different ion channels' influence on excitability.
Problem: Limitations of the patch-clamp technique restrict the conclusions which can be drawn from patch-clamp experiments
Aim: Develop in silico model of nociceptor soma to interpret the result from path-clamp experiments from the existing in silico C fiber model (Tigerholm et al. 2014)
Supervisors: Juni. Prof. Tigerholm
Project 2: In silico modelling of sodium channels
Background: Sodium channel mutations have been identified to lead to chronic pain syndromes. A specific sodium channel mutation can even lead to the inability to feel pain.
Problem: The existing in silico models of sodium channels need to be more sophisticated to study many of the pathological alterations occurring in patients.
Aim: Develop a Markov model of a sodium channel with an evolutionary optimization algorithm.
Supervisors: Juni. Prof. Tigerholm
Tigerholm J*, Petersson ME*, Obreja O, Esther Eberhardt, Namer B, Weidner C, Lampert A, Carr R, Martin Schmelz, Fransén E, 2015, C-Fiber Recovery Cycle Supernormality Depends on Ion Concentration and Ion Channel Permeability, 108:1057-71, Biophysical Journal, doi: 10.1016/j.bpj.2014.12.034, *Equal author contribution
Tigerholm J*, Petersson ME*, Obreja O, Lampert A, Carr R, Schmelz M, Fransen E, Modeling activity-dependent changes of axonal spike conduction in primary afferent C-nociceptors, Journal of Neurophysiology, 11:1721-35 2013, DOI: 10.1152/jn.00777.2012, *Equal author contribution
Poulsen AH, van den Berg B, Arguissain F, Tigerholm J, Buitenweg JR, Andersen OK, Mørch CD. Novel surface electrode design for preferential activation of cutaneous nociceptors. J Neural Eng., 19., 2022, doi: 10.1088/1741-2552/ac4950
Tigerholm J, Hoberg TN, Brønnum D, Vittinghus M, Frahm KS, Mørch CD, Small and large cutaneous fibers display different excitability properties to slowly increasing ramp pulses. J Neurophysiol. 124:883-894., 2020, doi: 10.1152/jn.00629.2019
Hugosdottir R, Mørch CD, Jørgensen CK, Nielsen CW, Olsen MV, Pedersen MJ, Tigerholm J, 2019, Altered excitability of small cutaneous nerve fibers during cooling assessed with the perception threshold tracking technique. 20:47, BMC Neurosci, doi: 10.1186/s12868-019-0527-3
Tigerholm J, Poulsen HA, Andersen KO, Mørch DC, 2019, From Perception Threshold to Ion Channels-A Computational Study, 117:281-295, Biophysical Journal. doi: 10.1016/j.bpj.2019.04.041
Poulsen AH, Tigerholm J, Meijs S, Andersen OK, Mørch CD., Comparison of existing electrode designs for preferential activation of cutaneous nociceptors. 108:1057-71, J Neural Eng. 17:036026. 2020, doi: 10.1088/1741-2552/ab85b1
Tigerholm J, Börjesson I S, Lundberg L, Elinder E, Fransén E, 2012, Dampening of Hyperexcitability in CA1 Pyramidal Neurons by Polyunsaturated Fatty Acids Acting on Voltage-Gated Ion Channels PLoS ONE, 7, doi: 10.1371/journal.pone.0044388
Fransén E, Tigerholm J, 2009, Role of A-type potassium currents in excitability, network synchronicity, and epilepsy, 20:877-87, Hippocampus. doi: 10.1002/hipo.20694
Maxion A, Kutafina E, Dohrn MF,.., Tigerholm J, Namer B. 2023, A modelling study to dissect the potential role of voltage-gated ion channels in activity-dependent conduction velocity changes as identified in small fiber neuropathy patients. Front Comput Neurosci, 17. doi:10.3389/fncom.2023.1265958



