Klump Lab
Generation of somatic stem and progenitor cells, in vitro
In all organisms, the constant replenishment of cells lost due to aging and tissue damage is guaranteed by somatic, tissue-resident stem cells. Because of their regenerative potential, these multipotent stem cells are prime targets for the treatment of a plethora of disorders. Our lab tries to understand how blood-forming stem cells (hematopoietic stem cells, HSCs) can be generated, in vitro, for future tailored cell- and gene therapy of patients
Development of hematopoietic stem- and progenitor cells from pluripotent stem cells, in vitro
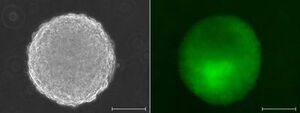
Pluripotent stem cells, such as embryonic stem (ES-) or `reprogrammed´, induced pluripotent stem (iPS-) cells, are defined by their capability to develop towards cells of all three germ layers, ecto-, meso- and endoderm. In contrast to the mesodermal, multipotent HSCs, these cells can be stably propagated in culture and are relatively easily amenable to genetic intervention by homologous recombination. Hence, patient-specific `autologous´ iPS-cells are especially attractive for gene repair and subsequent directed differentiation towards HSCs, in vitro. Because differentiation of pluripotent stem cells appears to autonomously recapitulate many aspects of the developing embryo, this model is also very useful for studying the control of cell fate decisions crucial for blood stem cell development, in vitro. We are studying in vitro hematopoiesis using pluripotent stem cells from mice and humans.
Funded by the Deutsche Forschungsgemeinschaft (DFG)
The homeodomain transcription factor HOXB4 and its impact on early hematopoietic progenitor development
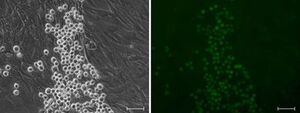
To generate multipotent hematopoietic stem- and progenitor cells (HSPCs), in vitro, we take advantage of the homeodomain transcription factor HOXB4, which supports hematopoietic development of differentiating pluripotent stem cells (such as ES- or iPS-cells) when expressed ectopically and also mediates expansion of adult HSPCs, in vitro and in vivo. A deeper understanding of the molecular pathways influenced by HOXB4 during pluripotent stem cell differentation will help us to substantially improve protocols for the in vitro generation of HSPCs and, thus, allow us to omit ectopically expressed supportive transcription factors in future.
Deciphering the mechanism(s) of HOXB4 action during hematopoietic development and expansion of HSPCs
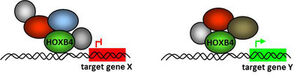
The molecular mechanisms how homeodomain transcription factors control self renewal and differentiation of hematopoietic stem and progenitor cells is still far from being clear. Although constitutive ectopic HOXB4 expression biases hematopoietic differentiation towards myelopoiesis and away from lymphopoiesis, it mediates a `benign´ HSC expansion without leading to leukemia, as observed with other HOX proteins, such as HOXB3 or HOXA10.
We have demonstrated that HOXB4 alters the sensitivity of many signaling pathways by changing the regulation of key genes involved in these processes. In turn, the activity of HOXB4 (i.e. transcriptional activation or repression of its target genes) appears to depend on the cell type itself and its context, the (micro)environment.
To gain a better understanding of the activities of this versatile transcription factor on HSC formation, self renewal and differentiation, we are identifying and studying its posttranslational modifications, protein interaction partners and their influence on target gene binding and expression.
Former lab-members (Master, M.D.- and Ph.D.-students)
- Susanne Skibbe (Technician)
- Mathias Manzel (Ph.D. student)
- Anika Neureiter (until 2019)
- Nadine Teichweyde (until 2017)
- Hannah Döpper (until 2018)
- Lara Kasperidus (until 2016)
- Julia Ulbricht (until 2017)
- Melanie Zuk (until 2015)
- Martina Cremanns (until 2017)
- Corinna Meyer (until 2014)
- Jana Rückforth (until 2021)
- Kristin Stolp (until 2014)
Peer Reviewed Publications:
Röth A, Bertram S, Schroeder T, Haverkamp T, Voigt S, Holtkamp C, Klump H, Wörmann B, Reinhardt HC and Alashkar F (2022) Acquired aplastic anemia following SARS-CoV-2 vaccination.
Eur J Haematology, 109, 186-194.
https://doi.org/10.1111/ejh.13788
Jaffredo T, Balduini A, Bigas A, Bernardi R, Bonnet D, Canque B, Charbord P, Cumano A, Delwel R, Durand C, Fibbe W, Forrester L, de Francheschi L, Ghevaert C, Gjertsen B, Göttgens B, Graf T, Heidenreich O, Hermine O, Higgs D, Kleanthous M, Klump H, Kouskoff V, Krause D, Lacaud G, Celso CL, Martens JHA, Méndez-Ferrer S, Menendez P, Oostendorp P, Philipsen S, Porse B, Raaijmakers M, Robin C, Stunnenberg H, Theilgaard-Mönch K, Touw I, Vainchenker W, Corrons JV, Yvernogeau L and Shuringa JJ (2022) The EHA Research Roadmap: Normal Hematopoiesis.
Hemasphere, 5, e699.
https://doi.org/10.1097/HS9.0000000000000669
Lindemann M, Klisanin V, Thümmler L, Fisenkci N, Tsachakis-Mück N, Ditschkowski M, Schwarzkopf S, Klump H, Reinhard HC, Horn PA and Koldehoff M (2021) Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients.
Vaccines, 9, 1075.
https://doi.org/10.3390/vaccines9101075
Proske P, Distelmaier L, Aramayo-Singelmann C, Koliastas N, Iannaccone A, Papthanasiou M, Temme C, Klump H, Lenz V, Koldehoff M, Carpinteiro A, Reinhardt HC, Köninger A, Röth A, Yamamoto R, Dührsen U and Alashkar F (2021) Pregnancies and Neonatal Outcomes in Patients with Sickle Cell Disease (SCD): Still a (High-)Risk Constellation?
J Pers Med, 11, 870.
https://doi.org/10.3390/jpm110908702
Lindemann M, Lenz V, Knop D, Klump H, Alt M, Aufderhorst U, Schipper L, Schwarzkopf S, Meller L, Steckel N, Koldehoff M, Heinold A, Heinemann F, Fischer J, Hutschenreuter G, Knabbe C, Dolff S, Brenner T, Dittmer U, Witzke O, Herbstreit F, Horn P and Krawczyk A (2021) Convalescent plasma treatment of critically ill intensive care COVID-19 patients.
Transfusion, 61, 1394-1403.
https://doi.org/10.1111/trf.16392
Lindemann M, Krawczyk A, Dolff S, Konik M, Rohn H, Platte M, Thümmler L, Schwarzkopf S, Schipper L, Bormann M, van de Sand L, Breyer M, Klump H, Knop D, Lenz V, Temme C, Dittmer U, Horn PA and Witzke O (2021) SARS-CoV-2-specific humoral and cellular immunity in two renal transplant and two haemodialysis patients treated with covalescent plasma.
J Med Virol 93, 3047-3054.
https://doi.org/10.1002/jmv.26840
Schwarzkopf S, Krawczyk A, Knop D, Klump H, Heinold A, Heinemann FM, Thümmler L, Temme C, Breyer M, Witzke O, Dittmer U, Lenz V, Horn PA and Lindemann M (2021) Cellular Immunity in COVID-19 Convalescents with PCR-Confirmed Infection but with Undetectable SARS-CoV-2-Specific IgG. Emerg Infect Dis 27, Epub 2020, Oct15.
https://doi.org/10.3201/2701.203772
Steens J, Unger K, Neureiter A, Wieber K, Hess J, Jakob HG, Klump H and Klein D (2019) Direct conversion of human fibroblasts into therapeutically active vascular wall-typical mesenchymal stem cells. Cell Mol Life Sci 77, 3401-3422.
https://doi.org/10.1007/s00018-019-03358-0
Teichweyde N, Kasperidus L, Carotta S, Kouskoff V, Lacaud G, Horn PA, Heinrichs S and Klump H (2018) HOXB4 Promotes Hemogenic Endothelium Formation without Perturbing Endothelial Cell Development. Stem Cell Reports 10, 875-889.
https://doi.org/10.1016/j.stemcr.2018.01.009
Teichweyde N, Horn PA, and Klump H (2017) HOXB4 increases Runx1 expression to promote the de novo formation of Multipotent Hematopoietic Cells. Transfus Med Hemother 44, 128-134.
https://doi.org/10.1159/000477130
Steens J, Zuk M, Benchellal M, Bornemann L, Teichweyde N, Hess J, Unger K, Görgens A, Klump H and Klein D (2017) In Vitro Generation of Vascular Wall-Resident Multipotent Stem Cells of Mesenchymal Nature from Murine Induced Pluripotent Stem Cells. Stem Cell Reports 8, 919-932.
https://doi.org/10.1016/j.stemcr.2017.03.001
Stanurova J, Neureiter A, Hiber M, de Oliveira Kessler H, Stolp K, Goetzke R, Klein D, Bankfalvi A, Klump H and Steenpass L (2016) Angelman Syndrome-derived neurons display late onset of paternal UBE3A silencing. Sci Rep 6, 30792.
https://doi.org/10.1038/srep30792
von Wnuck Lipinski K, Sattler K, Peters S, Weske S, Keul P, Klump H, Heusch G, Göthert JR and Levkau B (2016) Hepatocyte Nuclear Factor 1A is a cell intrinsic transcription factor required for B cell differentiation and development in mice. J Immunol 196, 1655-1665.
https://doi.org/10.4049/jimmunol.1500897
Engert A, Balduini C, Brand A, Coiffier B, Cordonnier C, Döhner H, Duyvene de Wit T, Eichinger S, Fibbe W, Green T, de Haas F, Iolascon A, Jaffredo T, Rodeghiero F, Salles G, Schuringa JJ (2016) The European Hematology Association Roadmap for European Hematology Research: a consensus document.
section 1.9. Kouskoff V, Forrester L, Graf T, Klump H, Lacaud G, Menendez P, Mountford J. Reprogramming/induced pluripotent stem cells/embryonic stem cells. Haematologica 101, 115-208.
https://doi.org/10.3324/haematol.2015.136739
Pilat S, Carotta S, and Klump H (2013) Development of Hematopoietic Stem and Progenitor Cells from Mouse Embryonic Stem Cells, in vitro, Supported by Ectopic HOXB4 Expression. Methods Mol Biol 1029, 129-147.
https://doi.org/10.1007/978-1-62703-478-4_10
Klump H, Teichweyde N, Meyer C, and Horn PA (2013) Development of patient-specific hematopoietic stem- and progenitor cell grafts from pluripotent stem cells, in vitro. Curr Mol Med 13, 815-820.
https://doi.org/10.2174/1566524011313050012
Lesinski DA, Heinz N, Pilat S, Rudolph C, Jacobs R, Schlegelberger B, Klump H, Schiedlmeier B. (2012) Serum- and stromal cell-free generation of embryonic stem cell-derived hematopoietic cells, in vitro, capable of multilineage repopulation of immunocompetent mice. Stem Cells Transl Med 1, 581-591.
https://doi.org/10.5966/sctm.2012-0020
Warlich E, Kühle J, Cantz T, Brugman MH, Maetzig T, Galla M, Filipczyk AA, Halle S, Klump H, Schöler HR, Baum, C, Schroeder T, Schambach A. (2011) Lentiviral vector design and imaging approaches to visualize the early stages of cellular reprogramming. Mol Ther 19, 782-9.
https://doi.org/10.1038/mt.2010.314
Chan KM, Bonde S, Klump H, Zavazava N. (2008) Hematopoiesis and immunity of HOXB4 transduced embryonic stem cell-derived hematopoietic progenitor cells. Blood 111, 2953-61.
https://doi.org/10.1182/blood-2007-10-117366
Schiedlmeier B, Santos AC, Ribeiro A, Moncaut N, Lesinski D, Auer H, Kornacker K, Ostertag W, Baum C, Mallo M, Klump H. (2007) HOXB4´s road map to stem cell expansion. Proc Natl Acad Sci USA 104, 16952-57.
https://doi.org/10.1073/pnas.0703082104
Will E, Speidel D, Wang Z, Ghiaur G, Rimek A, Schiedlmeier B, Ostertag W, Baum C, Klump H. (2006) HOXB4 inhibits cell growth in a dose-dependent manner and sensitizes cells towards extrinsic cues. Cell Cycle 5, 14-22.
https://doi.org/10.4161/cc.5.1.2304
Pilat S, Carotta S, Schiedlmeier B, Kamino K, Mairhofer A, Will E, Modlich U, Beug H, Ostertag W, Steinlein P, Baum C, Klump H. (2005) HOXB4 Enforces Equivalent Fates of ES-Cell Derived and Adult Hematopoietic Cells. Proc Natl Acad Sci USA 102, 12101-06.
https://doi.org/10.1073/pnas.0505624102
Klump H, Schiedlmeier B, Baum C. Control of Self-Renewal and Differentiation of Hematopoietic Stem Cells: HOXB4 on the Threshold. (2005) Annals NY Acad Sci 1044, 1-10.
https://doi.org/10.1196/annals.1349.002
Kraunus J, Schaumann DHS, Meyer J, Modlich U, Fehse B, Brandenburg G, von Laer D, Klump H, Schambach A, Bohne J, Baum C. (2004) Self-inactivating retroviral vectors with improved RNA processing. Gene Therapy 11, 1568-78.
https://doi.org/10.1038/sj.gt.3302309
Warncke M, Vogt B, Ulrich J, von Laer MD, Beyer W, Klump H, Micheel B, and Sheriff A (2004) Efficient in vitro transduction of naive murine B cells with lentiviral vectors. Biochem Biophys Res Commun 318, 673-679.
https://doi.org/10.1016/j.bbrc.2004.04.057
Schiedlmeier B, Klump H, Will E, Arman-Kalcek G, Li Z, Wang Z, Rimek A, Friel J, Baum C, Ostertag,W. (2003) High level ectopic HOXB4 expression confers a profound in vivo competitive growth advantage to human cord blood CD34+ cells, but impairs lymphomyeloid differentiation. Blood 101, 1759-68.
https://doi.org/10.1182/blood-2002-03-0767
Will E, Klump H, Heffner N, Schwieger M, Schiedlmeier B, Ostertag W, Baum C, Stocking C (2002) Unmodified Cre recombinase crosses the membrane. Nucl Acids Res 30, e59.
https://doi.org/10.1093/nar/gnf059
Klump H, Schiedlmeier B, Vogt B, Ryan M, Ostertag W, Baum C (2001) Retroviral vector-mediated expression of HoxB4 in hematopoietic cells using a novel coexpression strategy. Gene Therapy 8, 811-17.
https://doi.org/10.1038/sj.gt.3301447
Fehse B, Richters A, Putimtseva-Scharf O, Klump H, Li Z, Ostertag W, Zander AR, and Baum C (2000) CD34 splice variant: an attractive marker for selection of gene-modified cells. Molecular Therapy 1, 448-456.
https://doi.org/10.1006/mthe.2000.0068
Klump H, Auer H, Liebig HD, Kuechler E, and Skern T (1997) Proteolytically active 2A proteinase of human rhinovirus 2 is toxic for Saccharomyces cerevisiae but does not cleave the homologues of eIF-4 gamma in vivo or in vitro. Virology 220, 109-118.
https://doi.org/10.1006/viro.1996.0291
Sommergruber W, Ahorn H, Klump H, Seipelt J, Zoephel A, Fessl F, Krystek E, Blaas D, Kuechler E, Liebig HD, and Skern T (1994) 2A proteinases of coxsackie-
and rhinovirus cleave peptides derived from eIF-4 gamma via a common recognition motif. Virology 198, 741-745.
https://doi.org/10.1006/viro.1994.1089
Lamphear BJ, Yan R, Yang F, Waters D, Liebig HD, Klump H, Kuechler E, Skern T, and Rhoads RE (1993) Mapping the cleavage site in protein synthesis initiation factor eIF-4 gamma of the 2A proteases from human Coxsackievirus and rhinovirus. J Biol Chem 268, 19200-192003.
http://www.jbc.org/content/268/26/19200.full.pdf
Liebig HD, Ziegler E, Yan R, Hartmuth K, Klump H, Kowalski H, Blaas D, Sommergruber W, Frasel L, Lamphear B, Rhoads RE, Kuechler E, and Skern T (1993) Purification of two picornaviral 2A proteinases: interaction with eIF-4 gamma and influence on in vitro translation. Biochemistry 32, 7581-7588.
https://pubs.acs.org/doi/pdf/10.1021/bi00080a033
Book Chapters:
Klump H (2020) Hematopoietic Stem Cells. In: Brand-Saberi B, editor. Essential Current Concepts in Stem Cell Biology, Learning Materials in Biosciences, Switzerland: Springer Nature Group. p 1-21.
https://doi.org/10.1007/978-3-030-33923-4