GYM
Development of a 3D-printed in vitro-vessel model for the improvement of efficacy of stem cell therapies for muscular dystrophy
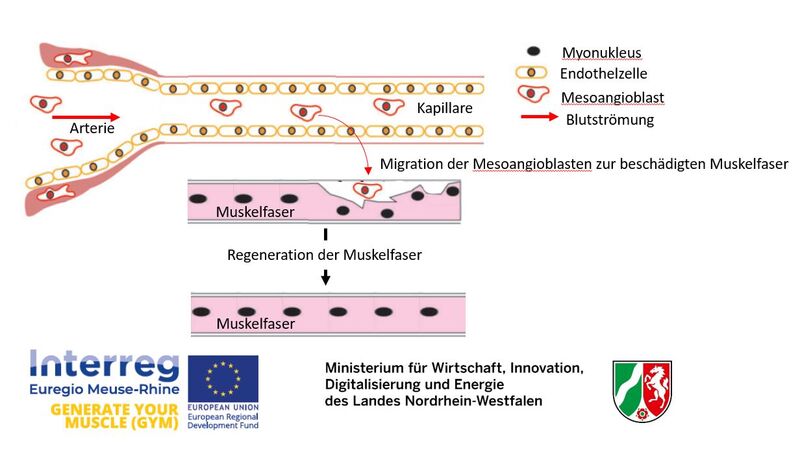
Muscular dystrophy is a genetically predisposed, degenerative disease where the patients are suffering under progressing muscular weakness. Consequences of this disease are amongst others an early immobility as well as a reduced life expectancy and quality of life. A stem cell therapy holds the potential to treat this disease and to facilitate a longer and qualitatively better life for the patients. The basic idea of the therapy comprises the injection of mesoangioblasts, which are mesenchymal cells located within the vessel walls, into the arterial system of the patients. The mesoangioblasts are able to migrate from the vessels through the vessel walls into the surrounding muscle tissue. Inside the muscle tissue, the mesoangioblasts evidently facilitate the regeneration and formation of muscle cells and fibers. Currently, only a small fraction of the injected mesoangioblasts reaches the targeted muscle tissue – the remaining stem cells are trapped inside the filter organs (liver, lungs, splenic).
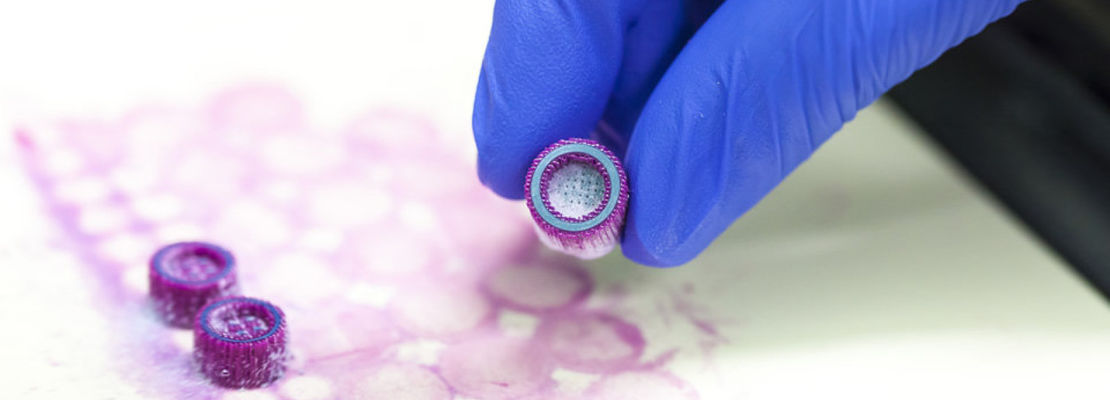
Within the by the European Union (Interreg Euregio Meuse-Rhine) funded interregional project GYM ('Generate Your Muscle') we work closely together with partners from the Netherlands (Clinical Genomics, School MHeNS, University of Maastricht and Scannexus) and Belgium (Stem Cell Institute, University of Leuven; Exercise Physiologiy and Sports Medical Center of Biomed, University of Hasselt; Laboratory of Hematology and Gene Therapy, University of Liège) in order to develop the stem cell therapy and help the patients. The aim of our project part is to increase the fraction of the migrating mesoangioblasts and thereby to optimize the efficacy of the stem cell therapy. Using a 3D bioprinter, an in vitro-vessel model will be developed, which will be used to both study and improve the mechanisms and kinetics of the migration of mesoangioblasts. Artificial blood vessels are printed and developed and mesoangioblasts are injected into the models afterwards to mimic the in vivo situation. Important aspects comprise the choice and characterization of a suitable hydrogel and the optimization of the bioprinting process. Subsequently, different growth factors and semiochemicals are investigated regarding their potential to improve the migration of the mesoangioblasts to the muscle tissue and thereby increase the efficacy of the stem cell therapy.