Our Interest
We are a team of young researchers aiming to understand how kidney diseases develop and how we could diagnose and treat them by using a broad array of approaches and methodologies, e.g. including non-invasive molecular imaging or AI-augmented diagnostics.
The main focus of our laboratory is on chronic kidney disease (CKD), the common end-point of virtually all renal diseases (progressive but also acute). CKD reached worldwide pandemic proportions with up to 10% of the world population being affected. Patients with CKD show a high risk of mortality and morbidity with survival rates in end-stage kidney disease compared to many malignancies.
We focus on the underlying pathological processes of CKD, such as renal fibrosis or vascular pathology and also cardiovascular consequences of CKD. Progressive renal fibrosis leads to loss of function and the need for renal replacement therapy (dialyzes or renal transplantation). At the moment we still lack both, sensitive diagnostic options as well as effective treatment therapies – both areas representing the main focuses of our research.
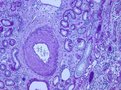
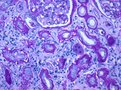
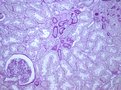
Left and middle pictures depict the histological appearance of renal fibrosis in patients with advanced kidney disease showing marked deposition of the extracellular matrix, vascular sclerosis, and associated tubular atrophy and chronic inflammatory infiltrate. For comparison, at the right, normal kidney parenchyma is shown with only a small area with interstitial fibrosis and tubular atrophy, a normal finding in aged kidneys.
Our group established a wide range of tools that allows us to comprehensively analyze pathological alterations in tissues. We consequently use a close translational approach, with a strong focus on clinical research whenever possible, and complement this with a wide array of in vitro and in vivo models. We continually extend and develop our broad methodological “toolbox” including multiscale imaging, from electron microscopy, histopathology to 3D and non-invasive and molecular imaging, new in vitro methodologies. We also have broad expertise in modeling kidney diseases in vivo, among others using cell-specific transgenic approaches, lineage tracing and in vivo imaging, and broad outcome analyses. We also use a wide variety of molecular and biochemical methods.
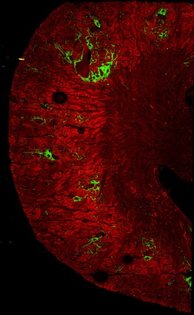
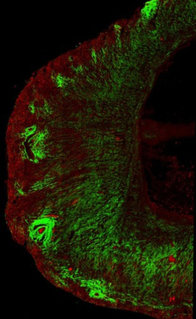
Murine kidneys showing collagen (in green) in the normal healthy kidney (left) and exaggerated deposition in the fibrotic kidney (right).
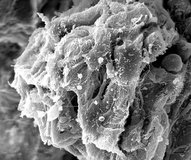
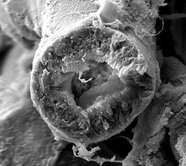
Scanning electron microscopy shows a glomerulus (left) and a normal-appearing proximal tubule cross-section (right).
A strong focus of our group is on the development of digital pathology, which is expected to transform pathology towards an innovative, reproducible, quantitative precision medicine field. We have established a comprehensive infrastructure allowing facilitated development of artificial intelligence (AI) and particularly machine learning (ML) and deep learning (DL) tools for preclinical as well as clinical histopathology (going beyond the kidney). A strong focus is on the development, testing, and validation of specifically tailored AI, and particularly DL algorithms for augmented diagnostics and data mining.
Finally, we have established and are leading the national Registry of COVID-19 Autopsies (DeRegCOVID), which facilitates (some aspects) of COVID-19 research and provide support and molecular testing of SARS-CoV-2 in pathology specimens.

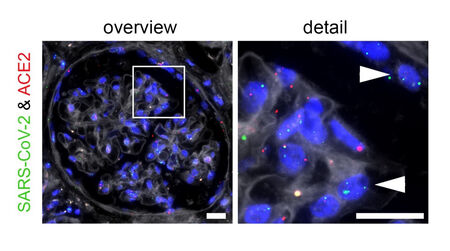
We are opened to collaborative projects and work closely with many researchers within the faculty, nationally and internationally.
Are you interested in knowing more about our projects, methods, models, and tools? Please contact us at pboorukaachende.





