Our Research
Developing proteomic-based approaches to study gut microbiota physiology in complex environments
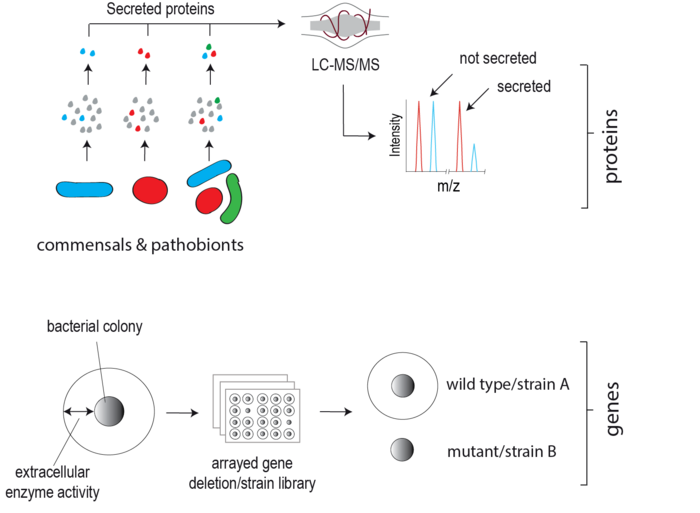
Extracellular proteins produced by gut microbes, unlike exo-metabolites, have remained largely understudied despite their clear potential to directly target and modify neighbouring microbes and the host. This lag is in part due to the inherent difficulty in detecting lowly abundant secreted microbial proteins among the dominant host background both in vitro and in vivo. We are developing state-of-the-art biochemical labelling and proteomic-based approaches to enrich, detect and quantify gut microbial secretomes within complex environments e.g. the mammalian gut. These approaches are facilitating the discovery of candidate microbiota secreted effector proteins. Our goal is to elucidate the physiological role of these proteins in host-microbe interplay using bacterial genetics, high-throughput phenotype screening and advanced biochemical methodologies.
Identifying and characterising clinically relevant proteases secreted by gut commensal bacteria
Proteolytic activity in the intestinal lumen plays a fundamental role in regulating mucosal barrier integrity, cytokine activity and availability, nutrient digestion, tissue remodelling and pathogen destruction. In addition to host-derived proteases, enteric pathogens also secrete proteases to combat host defence and serve as potent virulence factors. Interestingly, evidence is now emerging that human gut commensal bacteria (microbiota) also secrete proteases, yet their identity and roles in human physiology have not yet been explored in depth. We are applying genetic and biochemical methodologies to systematically identify and characterise proteases secreted by gut commensals linked to human disease e.g. Ulcerative Colitis (UC). Through genetic manipulation of protease-secreting microbes, we are studying the role of microbiota-secreted proteases in vitro and in vivo using Gnotobiotic rodents (the latter in close collaboration with the neighbouring Clavel lab).