Research Interest
The research interest in our group mainly focusses on the interaction between commensal and pathogenic microorganisms and the gut epithelium. The epithelium constitutes the luminal surface of the intestine and thus is situated in direct contact with the enteric microbiota and potentially pathogenic microorganisms. Originally, the main function of the epithelium was thought to be it’s ability to generate a physical barrier between the sterile host tissue and the microbially colonized gut lumen. Today, we know that epithelial cells actively contribute to establish and maintain the host-microbial homeostasis. In addition, they contribute to evoke an early host response in the event of oral challenge with enteropathogenic microorganisms.
The interaction between microorganisms and the gut epithelium is based on the so called innate immune system. The innate immune system facilitates an immediate recognition and rapid response of the host to microbial challenge. Microbial recognition is facilitated by so called pattern recognition receptor (PRR) molecules. PRRs constitute a heterogenous group of molecules that recognize evolutionary conserved microbial structures such as lipoolysaccharide from gram-negative bacteria and survey the cell surface, the cytosol but also intracellular compartments. Following ligand binding PRRs activate antimicrobial mechanisms of the innate immune system but also support activation of the adaptive immune response. One important group of innate immune effector molecules are antimicrobial peptides. These short proteins represent endogenous „antibiotics" are able to kill bacteria, some viruses and even parasites.
Since a couple of years, we have gained increasing interest in the situation in the neonate intestine during the postnatal period. In utero, the intestine is sterile and devoid of bacteria. With rupture of the membranes and birth, the neonate is exposed to maternal and environmental bacteria that rapidly colonize the neonate’s skin and mucosal surfaces. Interestingly, the neonate does not evoke an innate immune activation in response to postnatal microbial exposure and the mechanisms that contribute to the establishment of a stable host-microbial homeostasis and a diverse and stable enteric microbiota are largely undefined.
Also, several enteropathogenic microorganisms selectively target neonates and young infants but fail to evoke clinical disease or induce a completely different clinical picture in adult individuals. This suggests that the interaction between microorganisms and the host might fundamentally differ between the neonate and adult host. As a consequence, neonates might also deserve a different clinical managment. A better understanding of the innate and adaptive immune system in neonates might help to improve the current antiinfectious and antiinflammatory therapeutic options.
In addition, the immediate postnatal period appears to represent a „window of opportunity" for the development and maturation of the immune system. It might thus have lasting consequences on the host-microbial interaction also during adulthood. First evidence supports the notion that a „priming" of the immune system occurs after birth that significantly alters host-microbial interaction and influences the susceptibility to immune-mediated diseases such as allergies.
Innate immune receptor molecules facilitate the recognition of conserved microbial structures. Ligand binding activates the release of immune mediators and thereby the recruitment of professional immune cells as well as the production of antimicrobial effector molecules. In the event of a local infection, this can lead to the immediate elimination of the invading microorganism and prevent further microbial spread and systemic infection. On the other hand, an inappropriate and uncontrolled stimulation of the innate immune system induces an inflammatory host response and can potentially aggravate disease-associated tissue destruction. Also intestinal epithelial cells that generate the physical barrier between the largely sterile host tissue and the microbially colonized gut lumen express a variety of innate immune receptor molcules (Pott et al., 2012). This anatomical localization requires a tight control of innate immune stimulation to avoid an inappropriate response tot he enteric microbiota that may lead to chronic inflammation and tissue dysfunction.
One focus in our laboratory deals with the molecular basis, functional role and regulation of intestinal epithelial innate immune recognition. Starting point of our work was the analysis of a differentiated and polarized intestinal epithelial cell line that expressed the Toll-like receptor (TLR)4 and exhibited a surprising susceptibility to the ligand lipopolysaccharide (LPS) produced by gram-negative bacteria (Hornef et al., 2002). Further analyses revealed interesting differences in the cellular mechanisms of LPS recognition between myeloid and epithelial cells (Hornef et al., 2003). The functional importance of ligand internalization for epithelial activation was later confirmed (Duerr et al., 2009; Chassin et al., 2010). Tlr4 signals via the adaptor molecule MyD88 and the interleukin 1 associated kinase (IRAK) 1. The levels of IRAK1 protein were previously shown to significantly influence the cellular responsiveness. Interestingly, IRAK1 protein levels rise in the adult epithelium following transient ischemia due to SUMOylation and a change in the ubiquitination (K48 to K63) pattern. This increase is functionally relevant and contributes to the postischemic tissue damage (Chassin et al., EMBO Mol Med, 2012).
Also the double stranded RNA receptor Tlr3 is found at the intestinal epithelium. Tlr3 induces the production of type I (α/β) and III (λ) interferon. The functional relevance of TLR3 expression is illustrated by the enhanced susceptibility to rotavirus infection in adult mice deficient in TLR3 or the adaptor molecule TRIF (Pott et al., 2012). Virus infection of epithelial cells induces local expression of interferons; among them both type I (α/β) and interferon-λ. Strikingly, interferon-λ appears to specifically protect the epithelium leading to significantly enhanced rotavirus replication in interferon-λ receptor (IL-28R) deficient animals. In contrast, type I interferon stimulates antiviral protection of lamina propria cells (Pott et al., 2011).
Epithelial cells also express Nod2, the receptor for muramyl di-peptide (MDP), a molecular motif of the peptidoglycan layer of the outer bacterial cell wall. Secretion of the amidase PGLYRP-2 by intraepithelial lymphocytes reduces the stimulatory potential of peptidoglycan released by the enteric microbiota (Duerr et al., Muc Immunol., 2011). Nod2 recognizes epithelial infection with Listeria monocytogenes, although only after lysis of the endosomal membrane and entry to the epithelial cytosol. Strikingly, although innate immune stimulation is restricted to the infected cells, mainly the surrounding non-infected cells respond to the infection with cytokine secretion. The mechanism of this epithelial cell-cell communication was shown to rely on NADPH oxidase 4 (NOX4) mediated reactive oxygen species (ROS) (Dolowschiak et al., PLoS Pathogens, 2010) facilitating a coordinated epithelial response to focal infection.
Oral infection of neonate mice with the non-typhoid Salmonella enterica results in rapid colonization due to the absence of a diverse and competitive enteric microbiota. Surprisingly, we observed Salmonella pathogenicity island (SPI)1-dependent enterocyte invasion and the generation of intraepithelial microcolonies. Salmonella caused a potent epithelial innate immune activation that required cellular invasion and was mediated by Tlr4 and Tlr9 (Zhang et al., 2014).
Selected publications
Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002; 195(5): 559-70.
Hornef MW, Normark BH, Vandewalle A, Normark S. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med. 2003; 198(8): 1225-35.
Duerr CU, Zenk SF, Chassin C, Pott J, Gütle D, Hensel M, Hornef MW. O-antigen delays lipopolysaccharide recognition and impairs antibacterial host defense in murine intestinal epithelial cells. PLoS Pathog. 2009 ;5(9): e1000567.
Dolowschiak, T., Chassin, C., Ben Mkaddem, S., Fuchs, T.M., Weiss, S., Vandewalle, A., Hornef, M.W.. Potentiation of epithelial innate host responses by intercellular communication. PLoS Pathogens 6: e1001194, 2010.
Pott, J., MahlakoÞiv, T., Mordstein, M., Duerr, C.U., Michiels, T., Stockinger, S.*, Staeheli, P.*, Hornef, M.W.*. IFN- determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci U S A. 108: 7944-9, 2011.
Chassin C, Hempel C, Stockinger S, Dupont A, Kübler JF, Wedemeyer J, Vandewalle A, Hornef MW. MicroRNA-146a-mediated downregulation of IRAK1 protects mouse and human small intestine against ischemia/reperfusion injury. EMBO Mol Med. 4(12):1308- 19, 2012
Fulde, M., Hornef, M.W. Maturation of the mucosal innate immune system during the postnatal period. Rev Immunol. 260: 21-34, 2014.
Stockinger, S., Duerr, CU., Fulde,, M., Dolowschiak, T., Pott, J., Yang, I., Eibach, D., Bäckhed, F., Akira, S., Suerbaum, S., Brugman, M., Hornef, M.W. TRIF Signaling Drives Homeostatic Intestinal Epithelial Antimicrobial Peptide Expression. J Immunol. 193: 4223-34, 2014.
The immediate host defence of all multicellular organisms including mammals relies to a great extent on the production and targeted secretion of antimicrobial peptides, endogenously produced antibiotics. These small molecules inhibit a wide spectrum of microbial organisms such as bacteria, viruses, fungi, and protozoa and additionally possess potent anti-inflammatory activity facilitating bacterial killing in the absence of significant innate immune stimulation. Antimicrobial peptides in the gastrointestinal tract are produced by epithelial cells and Paneth cells situated at the basis of the small intestinal crypts.
Our work on epithelial antimicrobial peptides led to the characterization of a novel, large family of enteric antimicrobial peptides named cryptdin-related sequence (CRS) peptides. Similar to the established large group of enteric a -defensins, CRS peptides are expressed by Paneth cells and are released into the crypt lumen upon proteolytic processing. Expression analysis and representative cloning revealed 17 members of this novel peptide family in intestinal tissue. Synthesized CRS peptides showed broad antibacterial activity and rapid bacterial killing in the absence of detectable eukaryotic cytotoxicity. Mass spectrometric analysis demonstrated the presence of covalent dimeric peptides in various homo-, and heterodimeric combinations in vivo. CRS peptides are the first family to be shown to form covalent dimers, a strategy that might significantly expand the antimicrobial spectrum of the intestinal host defence (Hornef et al.; 2004).
Recent results suggest that homeostatic epithelial innate immune signalling in the adult host supports the development of enteric Paneth cells and the production of enteric antimicrobial peptides (Stockinger et al., 2014a). Furthermore, secretion by Paneth cells is induced by the parasympaticomimetic acetylcholine and Nod2 ligand muramyl-dipeptide (MDP). In addition, we demonstrated that also endogenous mediators such as IL-4 and IL-13 directly act on Paneth cells and induce degranulation and the secretion of antimicrobial peptides (Stockinger et al., 2014b).
Secreted antimicrobial peptides appear not to diffuse into the intestinal lumen but rather remain attached to the intestinal mucus layer as illustrated by the presence of antimicrobial peptides within isolated mucus material and the high mucus associated antibacterial activity (Meyer-Hoffert et al., 2008). The presence of antimicrobial peptides within the mucus layer generates a physicochemical shield and restricts bacterial penetration and innate immune stimulation by microbiota-derived immunostimulatory molecules (Dupont et al., 2014).
The neonate intestinal epithelium in mice lacks the formation of intestinal crypts and consequently also the presence of Paneth cells. In contrast, neonatal intestinal epithelial cells produce the cathelicidin related antimicrobial peptide (CRAMP). CRAMP expression wanes with the emergence of crypt Paneth cells two weeks after birth and the production of Paneth cell-derived antimicrobial peptides such as CRS peptides and a -defensins (Menard et al., 2008). This postnatal switch in the antimicrobial repertoire might support the establishment of a stable enteric microbiota after birth.
Current work on antimicrobial peptides focuses on the establishment of models to study the regulatory processes involved in the production, processing, storage, and secretion of these important enteric innate immune effector molecules. These studies aim to define the role of enteric antimicrobial peptides for the maintenance of the normal flora as well as hats defence during infection and may ultimately lead to the development of therapeutic strategies to induce endogenous antimicrobial peptide secretion and prevent or treat enteric microbial infection.
Selected Publications
Hornef MW, Putsep K, Karlsson J, Refai E, Andersson M. Increased diversity of intestinal antimicrobial peptides by covalent dimer formation. Nat Immunol. 2004 Aug;5(8):836-43.
Meìnard S, Förster V, Lotz M, Gütle D, Duerr CU, Gallo RL, Henriques-Normark B, Pütsep K, Andersson M, Glocker EO, Hornef MW. Developmental switch of intestinal antimicrobial peptide expression. J Exp Med. 2008; 205(1): 183-93.
Meyer-Hoffert U, Hornef MW, Henriques-Normark B, Axelsson LG, Midtvedt T, Pütsep K, Andersson M. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008; 57(6): 764-71.
Stockinger S, Albers T, Duerr CU, Meìnard S, Pütsep K, Andersson M, Hornef MW. Interleukin-
13-mediated paneth cell degranulation and antimicrobial peptide release. J Innate Immun. 2014;6(4):530-41.
Stockinger, S., Dolowschiak, T., Duerr, C.U., Pott, J., Eibach, D., Bäckhed, F., Suerbaum, S., Brugman, M., Hornef, M.W. TRIF determines intestinal epitelial homeostasis but is compensated during mucosal repair. J Immunol. 193: 4223-34, 2014.
Dupont A, Kaconis Y, Yang I, Albers T, Woltemate S, Heinbockel L, Andersson M, Suerbaum S, Brandenburg K, Hornef MW. Intestinal mucus affinity and biological activity of an orally administered antibacterial and anti-inflammatory peptide. Gut, 64: 222-32, 2015.
With rupture of the membranes and birth, microbial colonization of the skin and mucosal surfaces starts and rapidly leads to a dense and highly diverse microbial community, particularly in the lower gastrointestinal tract. Intestinal epithelial cells also in the neonate intestine express innate immune receptors and are able to stimulate immune activation. The mechanisms, that prevent an inappropriate stimulation during postnatal bacterial colonization are only incompletely understood.
We had made the striking observation of a transient period of reduced LPS susceptibility during the postnatal period after vaginal delivery. Reduced ligand susceptibility was caused by a sustained posttranscriptional down-regulation of epithelial interleukin 1 receptor associated kinase (IRAK)-1, an essential signaling molecule of TLR-mediated cell stimulation (Lotz et al., 2006). Both proteasomal/lysosomal degradation and microRNA mediated translational repression cause reduced epithelial IRAK1 protein levels after birth (Chassin et al., 2010). In contrast, IRAK1 levels normalize after weaning and epithelial TLR stimulation significantly contributes to homeostatic epithelial signalling (Stockinger et al., 2014). Thus, the acquired downregulation of IRAK1 protein levels appears to protect the neonate epithelium from excessive Tlr4-mediated immune stimulation induced by the emerging enteric microbiota.
An example for a developmentally regulated immune adaptation is the Blimp1-mediated reduction in the epithelial Tlr3 expression during the postnatal period. The functional relevance of low TLR3 expression in the neonate host is illustrated by the enhanced susceptibility to rotavirus infection in neonate mice (Pott et al., 2012).
Also the susceptibility to bacterial infection differs significantly between the adult and neonate host. In adult mice, orally administered non-typhoid Salmonella enterica mainly translocates through M cells overlaying the intestinal Peyer’s patches in order to spread to lymph node, spleen and liver tissue. Infected enterocytes can hardly be visualized. Oral infection of neonate mice with Salmonella results in rapid colonization due to the absence of a diverse and competitive enteric microbiota. In contrast to the situation in the adult host, we observed Salmonella pathogenicity island (SPI)1-dependent enterocyte invasion and the generation of readily detectable intraepithelial microcolonies. Salmonella invasion induced TLR4 and 9 stimulation and caused a potent epithelial innate immune activation with upregulation of inflammatory, metabolic and developmental genes (Zhang et al., 2014).
Also the adaptive immune system underlies significant maturation during the postnatal period. CD4+ TCRαβ+ T lymphocytes home to the intestinal Peyer’s patches only hours after birth. Despite the rapid establishment of the enteric microbiota, however, these cells remain restricted to Peyer’s patches and maintain immaturity. Both maternal IgA antibodies as well as neonatal regulatory T lymphocytes contribute to prevent immune maturation. This may help to preserve the T lymphocyte repertoire and avoid the generation of cross reactive T cells (Torow et al., 2015).
Selected publications
Lotz, M., Gütle, D., Meìnard, S., Walther, S., Bogdan, C., Hornef, M.W.. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006; 203(4): 973-84.
Chassin, C., Kocur, M., Pott, J., Duerr, C.U., Gütle, D., Lotz, M., Hornef, M.W. miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe 2010, 8: 358-68.
Pott J, Stockinger S, Torow N, Smoczek A, Lindner C, McInerney G, Bäckhed F, Baumann U, Pabst O, Bleich A, Hornef MW. Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathogens, 8(5):e1002670. 2012
Zhang, K., Dupont, A., Torow, N., Gohde, F., Leschner, S., Lienenklaus, S., Weiss, S., Brinkmann, M.M., Kühnel, M., Hensel, M., Fulde., M.*, Hornef, M.W. Age-dependent enterocyte invasion and microcolony formation by Salmonella. PLoS Pathog. 10(9): e1004385, 2014.
Torow, N., Yu, K., Hassani, K., Bleich, A., Lochner, M., Brenneke, A., Weiss, S., Förster, R., Pabst, O., Hornef, M.-W. Active suppression of intestinal CD4+TCRαβ+ T lymphocyte maturation during the postnatal period. Nat Commun. 6: 7725, 2015.
Dupont, A., Litvak, Y., Zhang, K., Bleich A., Fulde, M., Rosenshine, I., Hornef M.W. Oral enteropathogenic E. coli (EPEC) infection of newborn mice. PLoS Pathogens, doi: 10.1371/journal.ppat.1005616
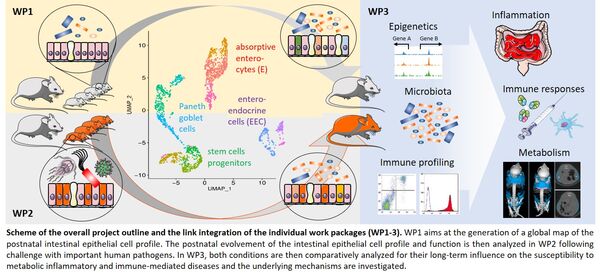
Infections of the gastrointestinal tract and their long-term consequences remain a major cause of childhood mortality and morbidity worldwide. In addition, early life is recognized as a critical and non-redundant time period to prime the mucosal tissue and establish the enteric microbiota that determine the risk to develop prevalent inflammatory, immune-mediated, and metabolic diseases. Three factors: the enteric microbiota, the mucosal immune system and the epithelial barrier cooperate to establish intestinal host-microbial homeostasis after birth. Maturation of the mucosal immune system and establishment of the enteric microbiota have been extensively studied. In contrast, postnatal evolvement of epithelial cell type heterogeneity and functional specialization and the influence of enteric infection on this process have not been explored. With EarlyLife, I propose to further advance innovative, multiscale technical approaches and analytical protocols in combination with novel in vivo models to generate the first comprehensive map of postnatal epithelial cell type and subtype differentiation and analyze the impact of early life infection by important human bacterial, viral and parasitic pathogens. Long-term inflammatory, immune-mediated and metabolic effects will be functionally studied using epigenetic profiling, microbiota-transfer experiments, stem cell organoid culture, and genetic models. Identified mechanisms will be confirmed using single-cell analysis of human mucosal biopsies, human stem cell organoids and transcriptomic profiling of human fecal samples. As a result, I expect to identify mechanisms of enhanced infection susceptibility of the neonate, decipher the critical and non-redundant influence of the postnatal period for mucosal homeostasis and explain the role of early life imprinting for long term immune-mediated, inflammatory and metabolic diseases.
Reviews zum Nachlesen
Reviews for further information
Renz, H, Brandtzaeg, P, Hornef, MW. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 12:9-23, 2011
Pott, J., Hornef, M.W. Innate immune signalling at the intestinal epithelium in homeostasis and disease. EMBO Rep, 13(8):684-98, 2012
Fulde, M., Hornef, M.W. Maturation of the mucosal innate immune system during the postnatal period. Rev Immunol. 260: 21-34, 2014
Dupont, A., Heinbockel, L., Brandenburg, K., Hornef, M.W. Antimicrobial peptides and the enteric mucus layer act in concert to protect the intestinal mucosa. Gut Microbes. 5: 761-5, 2014
Zhang K, Hornef MW, Dupont A. The intestinal epithelium as guardian of gut barrier integrity. Cell Microbiol. 17(11):1561-9, 2015.
van Best N, Hornef MW, Savelkoul PH, Penders J. On the origin of species: Factors shaping the establishment of infant's gut microbiota. Birth Defects Res C Embryo Today. 105(4):240-51, 2015.
Hornef MW, Pabst O. Real friends: Faecalibacterium prausnitzii supports mucosal immune homeostasis. Gut. 65(3):365-7, 2016.
Pabst, O., Cerovic, V., Hornef, M.W. Secretory IgA coordinates establishment and maintenance of the microbiota. Trends Immunol. pii: S1471-4906(16)00040-5, 2016.